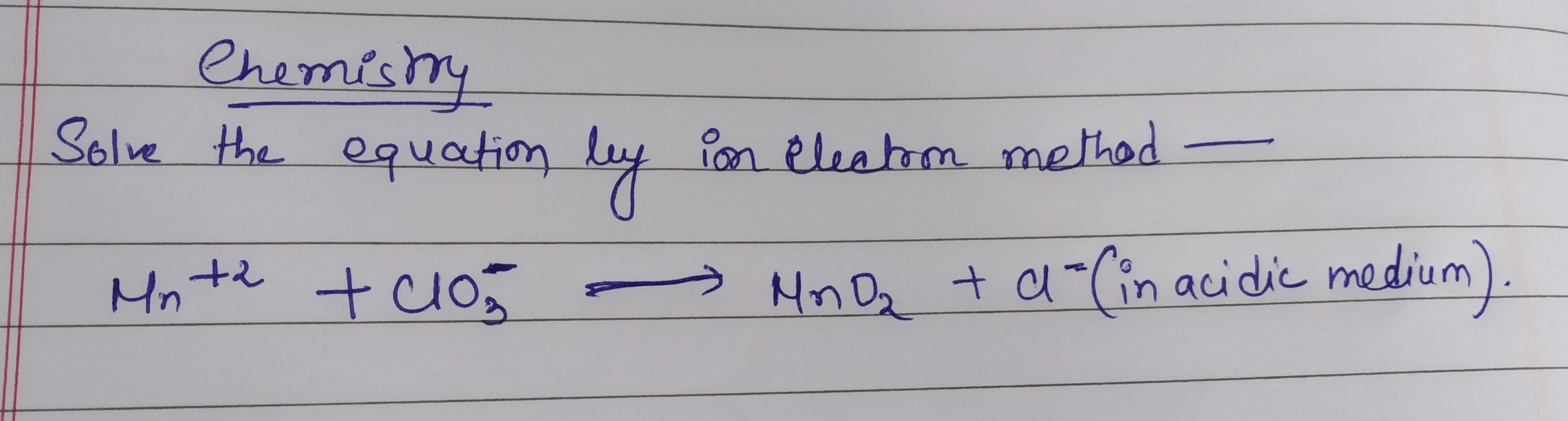
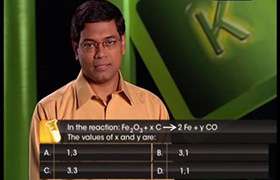CBSE Class 11-science Answered
H2O2+Fe2+=H2O +Fe3+ , give ion exchange method
Asked by Punshibakhuraijam2015 | 22 Sep, 2019, 08:40: PM
First half reaction
2e- +H2O2 +2H+ → 2H2O
To balance chare we have to add 2e-.Look now both sides have 0 charge.(2+ and 2- =0)
(Fe2+ = Fe3+ + e- ) × 2
Final equation is H2O2 +2H+ + 2Fe2+ → 2H2O + 2Fe3+
Answered by Ramandeep | 23 Sep, 2019, 11:45: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by indranibaudya | 03 Oct, 2020, 08:12: PM
CBSE 11-science - Chemistry
Asked by Punshibakhuraijam2015 | 22 Sep, 2019, 08:40: PM
CBSE 11-science - Chemistry
Asked by kkdmmdsd | 09 Jun, 2019, 04:27: PM
CBSE 11-science - Chemistry
Asked by dheerajmathpal374 | 15 Sep, 2018, 10:43: AM
CBSE 11-science - Chemistry
Asked by govtsecschoolnayaganv051 | 19 Aug, 2018, 06:29: PM
CBSE 11-science - Chemistry
Asked by govtsecschoolnayaganv051 | 18 Aug, 2018, 05:08: PM
CBSE 11-science - Chemistry
Asked by govtsecschoolnayaganv051 | 14 Aug, 2018, 06:06: PM
CBSE 11-science - Chemistry
Asked by vaagai2353 | 29 Jun, 2018, 10:29: PM
CBSE 11-science - Chemistry
Asked by g_archanasharma | 22 Mar, 2018, 10:11: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM