CBSE Class 11-science Answered
Great confusion...
Asked by abhinay.bhatta | 01 Mar, 2010, 06:57: PM
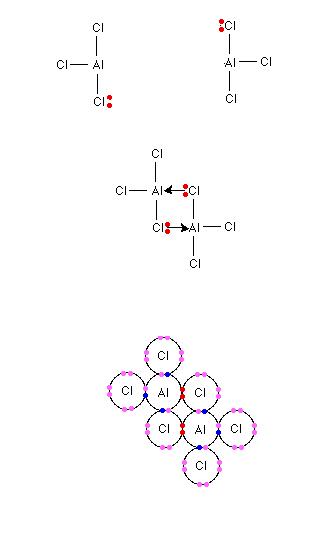
See, AlCl3 is a covalent compound. So no question of Al3+.
Al has 3 valence electron which it shares with 3 Cl atoms to form 3 Al - Cl bonds.
This molecule is electron deficient as it has only 6 valence electrons and not 8.
So, to complete its electron deficiency, one of the Cl donates an electron pair to be shared between Al and Cl. This bond is a coordinate bond.
SImilarly, a second Cl donates an electron pair to be shared between Al and Cl of the other molecule.
Thus, the structure is as:
Answered by | 02 Mar, 2010, 02:13: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by singhnikita8717 | 05 Aug, 2021, 10:44: AM
CBSE 11-science - Chemistry
Asked by jaswindernkd | 21 Jun, 2020, 05:04: PM
CBSE 11-science - Chemistry
Asked by mufeedatvp2000 | 18 Apr, 2020, 02:21: PM
CBSE 11-science - Chemistry
Asked by Molaypaul700 | 10 Feb, 2020, 10:32: PM
CBSE 11-science - Chemistry
Asked by ABHILASHA | 08 Sep, 2019, 06:46: PM
CBSE 11-science - Chemistry
Asked by krishdabhoya2003 | 05 Aug, 2019, 08:19: AM
CBSE 11-science - Chemistry
Asked by vishakhachandan026 | 12 Jun, 2019, 09:20: AM
CBSE 11-science - Chemistry
Asked by pb_ckt | 28 Apr, 2019, 01:40: PM
CBSE 11-science - Chemistry
Asked by satya785583 | 16 Mar, 2019, 09:18: PM
CBSE 11-science - Chemistry
Asked by govtsecschoolnayaganv051 | 03 Jan, 2019, 01:09: PM