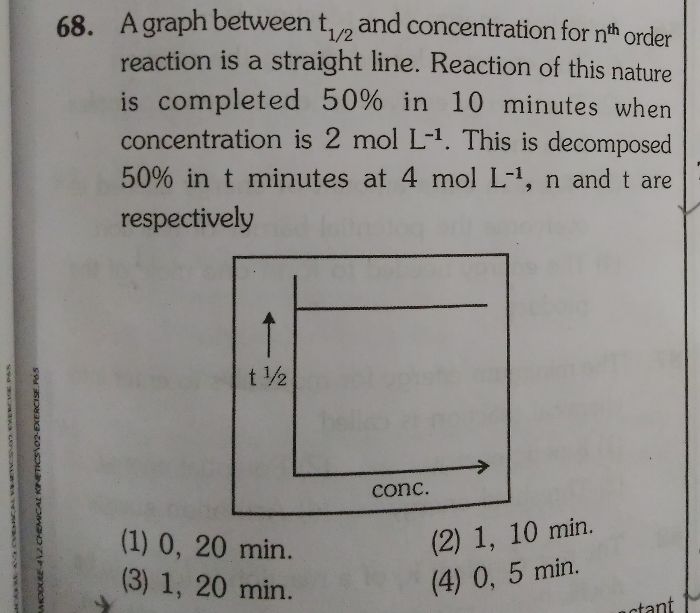
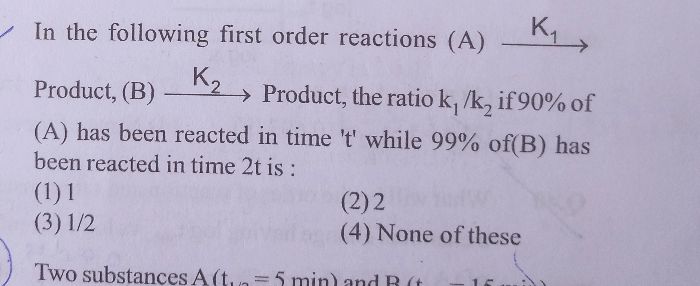CBSE Class 12-science Answered
Given reaction 2 NO2 + O2 --> N2O5 + O2. It is believed that a species with the formula NO3 is involved in the mechanism, and the observed rate law for the overall reaction is rate = k[NO2][O3]. Propose a mechanism for this reaction that includes the species NO3 and is N2O5 is consistent with the observed rate law.
Asked by | 08 Dec, 2012, 07:05: PM
The rate equation shows us how many atoms or molecules of a certain chemical is present in the slow step. In the above rate equation, it shows that only 1 NO2 and 1 O3 is involved in the slow step. Therefore, when choosing the answer, you should look answer which have only 1 NO2 molecule and 1 O3 molecule in the slow step.
NO2 + O3 => NO3 + O2 (slow)
NO3 + NO2 => N2O5 (fast)
NO3 + NO2 => N2O5 (fast)
Answered by | 21 Dec, 2012, 09:36: AM
Concept Videos
CBSE 12-science - Chemistry
Asked by bhadauriyax | 30 Nov, 2023, 06:23: PM
CBSE 12-science - Chemistry
Asked by rahulbiswal946 | 08 Nov, 2023, 07:01: PM
CBSE 12-science - Chemistry
Asked by mishraridhi2020 | 23 Jun, 2022, 09:16: AM
CBSE 12-science - Chemistry
Asked by cjam41665 | 10 Oct, 2021, 12:56: AM
CBSE 12-science - Chemistry
Asked by arshbhatia0809 | 22 Jul, 2021, 09:47: PM
CBSE 12-science - Chemistry
Asked by amitkumar.cis | 14 Jan, 2021, 12:40: PM
CBSE 12-science - Chemistry
Asked by Surendersingh0493 | 18 Oct, 2020, 02:05: PM
CBSE 12-science - Chemistry
Asked by khandarev3580 | 10 Oct, 2020, 10:54: AM
CBSE 12-science - Chemistry
Asked by rchaitra1204 | 07 Sep, 2020, 09:43: AM
CBSE 12-science - Chemistry
Asked by dr.akanksha0411 | 07 Aug, 2020, 11:56: AM