CBSE Class 12-science Answered
Copper is oxidized by concentrated nitric acid, HNO3, to produce Cu2+ ions; the nitric acid is reduced to nitrogen dioxide, a poisonous brown gas with an irritating odor:
Cu(s) + 4HNO3(aq) ——> Cu(NO3)2(aq) + 2NO2(g) + 2H2O(l)
When the copper is first oxidized, the solution is very concentrated, and the Cu2+ product is initially coordinated to nitrate ions from the nitric acid, giving the solution first a green, and then a greenish-brownish color. When the solution is diluted with water, water molecules displace the nitrate ions in the coordinate sites around the copper ions, causing the solution to change to a blue color.
In dilute nitric acid, the reaction produces nitric oxide, NO, instead:
3Cu(s) + 8HNO3(aq) ——> 3Cu(NO3)2(aq) + 2NO(g) + 4H2O(l)
Topperlearning Team.




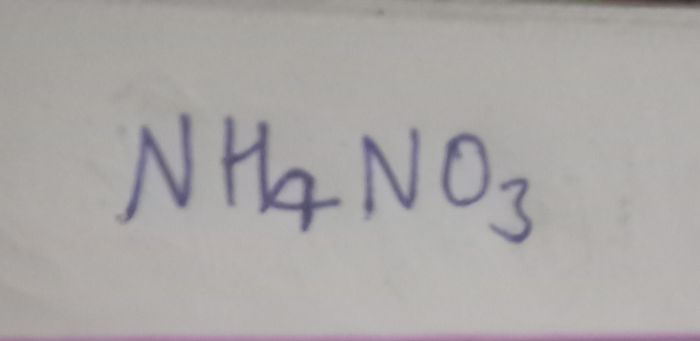
 to produce a brown coloured gass which intensifies on addition on copper turnings . on adding dilute ferrous sulphate olution to an aqueous solution to an aquaeous solution of X and then carefully adding conc.
to produce a brown coloured gass which intensifies on addition on copper turnings . on adding dilute ferrous sulphate olution to an aqueous solution to an aquaeous solution of X and then carefully adding conc. 