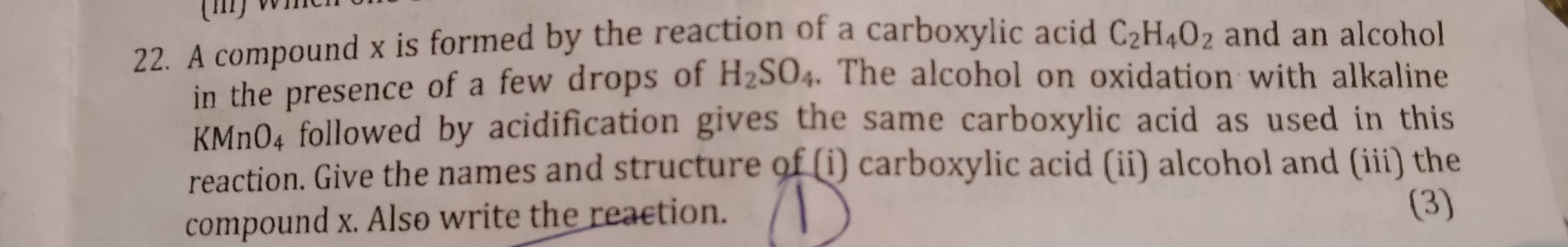CBSE Class 10 Answered
Give a test for experimentally distinguishing between an alcohol and a carboxylic acid and describe how these tests are performed?
Asked by Eshwer | 17 Mar, 2017, 12:22: AM
1) On addition of carbonates or bicarbonates to an aqueous solution of carboxylic acid, brisk effervescence is observed.
Metal carbonates/Bicarbonates + Carboxylic acid → Salt + Water + Carbon dioxide
Alcohol does not give this test.
2) On addition of sodium metal to alcohol, hydrogen gas is evolved.
Example:
2CH3CH2OH + 2Na → 2CH3CH2ONa + H2
Carboxylic acid does not give this test.
Answered by Prachi Sawant | 17 Mar, 2017, 09:29: AM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by sneh | 27 Mar, 2020, 10:11: AM
CBSE 10 - Chemistry
Asked by pranjaliinamdar2004 | 29 Feb, 2020, 07:20: PM
CBSE 10 - Chemistry
Asked by sweetykhatri99254 | 27 Feb, 2020, 03:40: PM
CBSE 10 - Chemistry
Asked by priyanshiishu | 30 Jan, 2020, 10:39: AM
CBSE 10 - Chemistry
Asked by kamalnayansingh7 | 13 Jan, 2020, 08:35: AM
CBSE 10 - Chemistry
Asked by Deepak | 22 Dec, 2019, 11:20: PM
CBSE 10 - Chemistry
Asked by ritikraghuwanshi6986 | 16 Dec, 2019, 08:42: PM
CBSE 10 - Chemistry
Asked by vedantsagrawal23 | 05 Dec, 2019, 08:34: AM
CBSE 10 - Chemistry
Asked by aryasaxena2003 | 25 Jul, 2019, 05:35: PM
CBSE 10 - Chemistry
Asked by rushabhjain.avv | 21 Mar, 2019, 10:07: PM