ICSE Class 9 Answered
find the percentage of nitrogen in urea nh2conh2 h=1 c=12 n=14 o=16
Asked by jaydevsahu4455 | 23 Mar, 2021, 10:33: AM
Molecular weight of NH₂CONH₂ is 60g and Molecular weight of N is 14.
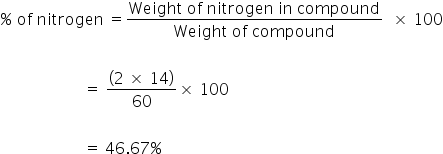
% of nitrogen in urea is 46.67%
Answered by Ramandeep | 23 Mar, 2021, 12:29: PM
ICSE 9 - Science
Asked by kalayat16 | 16 Apr, 2024, 03:00: PM
ICSE 9 - Science
Asked by vedantshrivastava18385 | 14 Dec, 2023, 06:49: AM
ICSE 9 - Science
Asked by sudarshanc757 | 08 Sep, 2023, 08:05: PM
ICSE 9 - Science
Asked by bharthiadarsh | 12 Aug, 2023, 07:23: PM
ICSE 9 - Science
Asked by yaganti2535 | 22 Jul, 2022, 07:40: PM
ICSE 9 - Science
Asked by Angadneb | 24 Apr, 2022, 11:15: AM
ICSE 9 - Science
Asked by najeedahamed87 | 07 Dec, 2021, 10:17: PM
ICSE 9 - Science
Asked by kc0112210 | 25 Oct, 2021, 09:44: PM
ICSE 9 - Science
Asked by paramjt58 | 14 Sep, 2021, 11:55: AM
ICSE 9 - Science
Asked by sohailbhai7859 | 03 Sep, 2021, 09:22: AM

