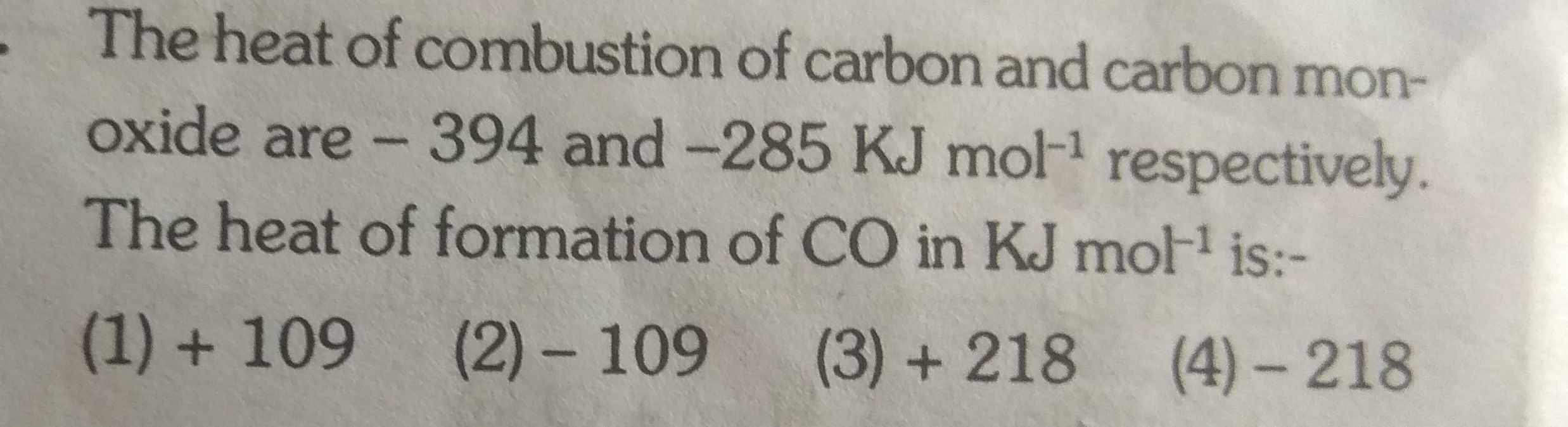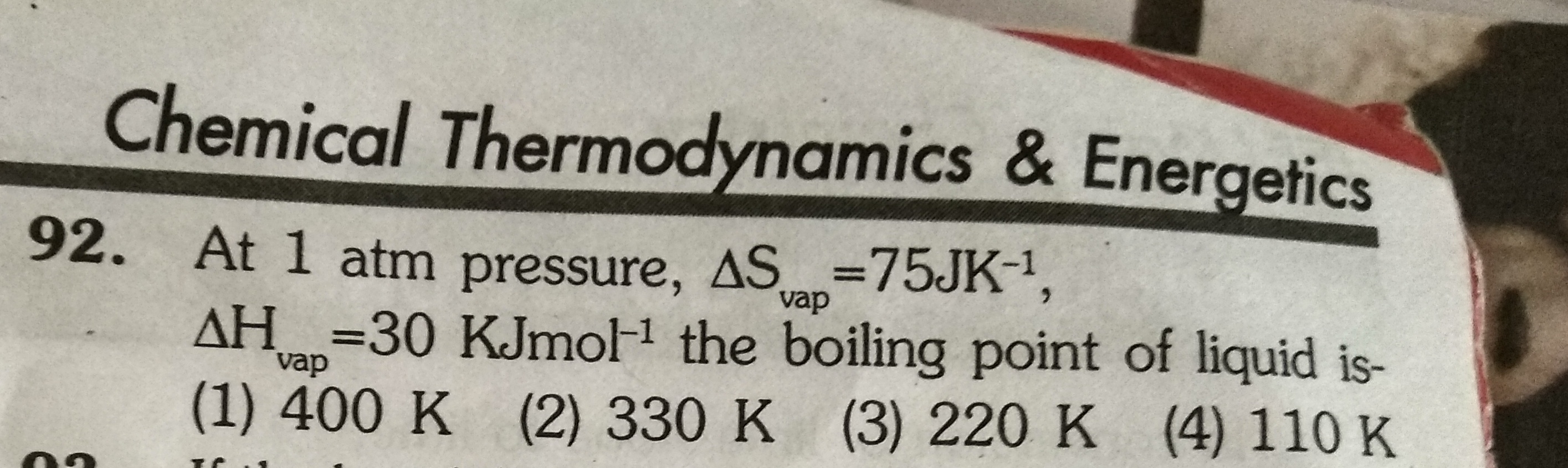JEE Class main Answered
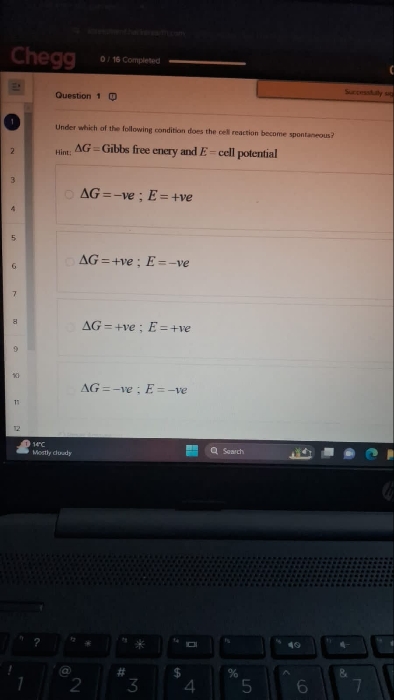
Find internal energy ?
Asked by ashutosharnold1998 | 31 Oct, 2019, 07:16: PM
Internal Energy:
Every substance possesses a definite amount of energy which depends on factors such as chemical nature, temperature and pressure.
This energy is called the internal energy of the system. It is represented by U.
The internal energy U of the system may change when:
Heat passes into or out of the system
Work is done on or by the system
DU = UB−UA
Where,
UB= internal energy of the system in State B
UA= internal energy of the system in State A
Internal energy of a system cannot be measured directly, but can determine the change in internal energy (ΔU)
Answered by Varsha | 01 Nov, 2019, 09:44: AM
JEE main - Chemistry
Asked by ashutosharnold1998 | 03 Nov, 2019, 08:22: PM
JEE main - Chemistry
Asked by ashutosharnold1998 | 31 Oct, 2019, 07:16: PM
JEE main - Chemistry
Asked by ashutosharnold1998 | 10 Aug, 2019, 12:10: AM
JEE main - Chemistry
Asked by Ranjeetgupta26068 | 19 May, 2019, 10:14: PM
JEE main - Chemistry
Asked by g_archanasharma | 20 Feb, 2019, 05:23: PM
JEE main - Chemistry
Asked by g_archanasharma | 08 Feb, 2019, 05:48: PM