CBSE Class 11-science Answered
Explain with reason...
Why aqueous HCl is more acidic than unhydrous HCl?
Asked by Ginni2k | 11 Nov, 2016, 09:43: PM
In aqueous medium, HCl is the strongest and anhydrous HCl is the weakest acid.
In the presence of water, HCl is entirely ionized with strong hydrogen bonding which is not in case of anhydrous HCl.
Answered by Vaibhav Chavan | 12 Nov, 2016, 11:38: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by Topperlearning User | 09 Oct, 2014, 07:06: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 09 Oct, 2014, 07:34: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 09 Oct, 2014, 07:42: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 13 Jun, 2016, 02:43: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 09 Oct, 2014, 08:36: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 13 Jun, 2016, 02:43: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 09 Oct, 2014, 08:42: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 09 Oct, 2014, 08:58: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 13 Jun, 2016, 02:43: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 13 Jun, 2016, 02:43: PM



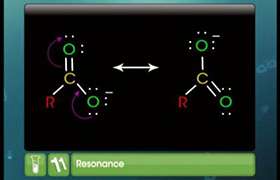
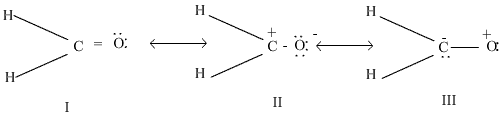 ii) What are the factors on which the dipole moment of polyatomic molecules depends?
ii) What are the factors on which the dipole moment of polyatomic molecules depends?