CBSE Class 11-science Answered
Explain why helium molecule does not exist?
Asked by Topperlearning User | 08 Oct, 2014, 01:56: PM
Helium atom (1s2) has 2 electrons in its 1s orbital.
During the approach of two helium atoms, the repulsive forces are dominant over the attractive forces. As a result the energy of the system increases which leads to instability.
Since energy of the separate helium atoms is smaller than that of the system when they are close to each other, they prefer to stay separate and do not form He2 molecule.
Answered by | 08 Oct, 2014, 03:56: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by Trisha Gupta | 30 Oct, 2022, 05:36: PM
CBSE 11-science - Chemistry
Asked by ABHILASHA | 22 Aug, 2020, 04:39: AM
CBSE 11-science - Chemistry
Asked by kpbhake | 12 Mar, 2018, 11:45: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 08 Oct, 2014, 01:09: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 13 Jun, 2016, 02:26: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 11-science - Chemistry
What is the hybrid state of B in BF3, Al in AlCl3, Be in BeCl2, C in CO2 and C2H4; S in SO2 and SO3.
Asked by Topperlearning User | 08 Oct, 2014, 01:33: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 09 Oct, 2014, 09:30: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM


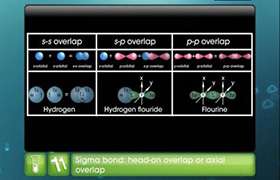
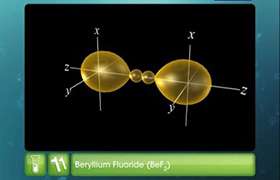
 on the basis of hybridisation
on the basis of hybridisation