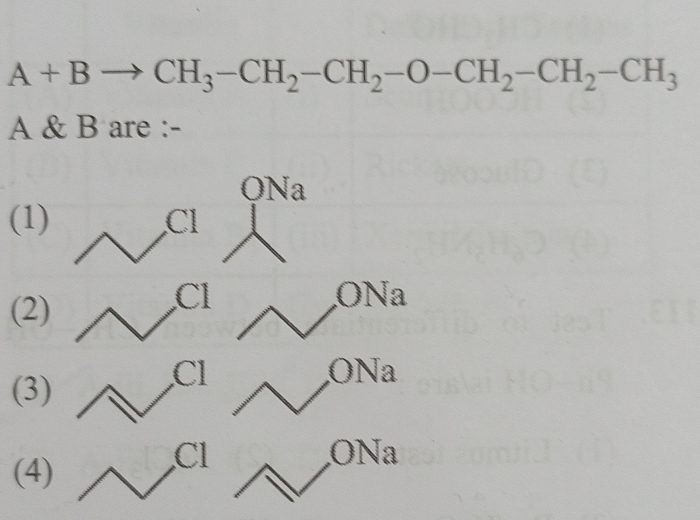
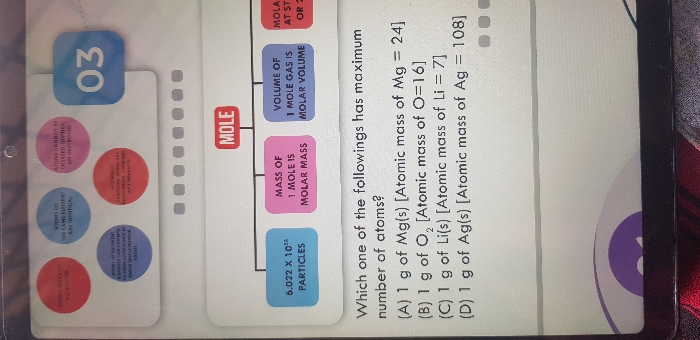
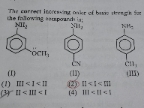
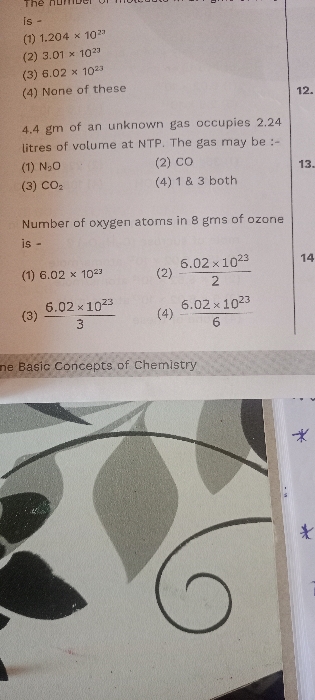NEET Class neet Answered
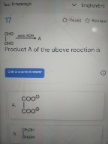
Explain this line with a suitable example

Asked by myindiaisbad | 02 Apr, 2023, 10:58: AM
Dear Student,
Inductive effect is distance dependent since it depends on the distance from electrophile or nuclephile. Inductive effect is shifting of sigma electrons.
While in case of electromeric effect, complete transfer of pi electrons occur which is independent of distance.
When inductive and electromeric effect operates in opposite direction, the electromeric effect predominates because it is strong effect.
Answered by | 03 Apr, 2023, 11:15: AM
Application Videos
Concept Videos
NEET neet - Chemistry
Asked by mahendar160786 | 16 Apr, 2024, 09:23: PM
NEET neet - Chemistry
Asked by raomayankup83 | 15 Apr, 2024, 07:46: PM
NEET neet - Chemistry
Asked by muskannawab11 | 14 Apr, 2024, 03:13: PM
NEET neet - Chemistry
Asked by 8239682116rahul | 10 Apr, 2024, 01:48: PM
NEET neet - Chemistry
Asked by tarasingrathod63 | 07 Apr, 2024, 01:07: PM
NEET neet - Chemistry
Asked by fathimahusna6122 | 05 Apr, 2024, 10:25: AM
NEET neet - Chemistry
Asked by vasantagomasi23 | 05 Apr, 2024, 08:35: AM
NEET neet - Chemistry
Asked by ankuruthanuriya | 03 Apr, 2024, 10:56: PM
NEET neet - Chemistry
Asked by ghousiakaneez | 03 Apr, 2024, 12:55: PM