CBSE Class 12-science Answered
Explain the working of simple voltaic cell?
Asked by | 09 Jul, 2008, 05:29: PM
votaic cell a simple device with which chemical energy is converted into electrical energy. Two dissimilar metals (e.g., copper and zinc) are immersed in an electrolyte (e.g., a dissolved sulfate). If the metals are connected by an external circuit, one metal is reduced (i.e., gains electrons) while the other metal is oxidized (i.e., loses electrons). In the example above, copper is reduced and zinc is oxidized. The difference in the oxidation potentials of the two metals provides the electric power of the cell
Answered by | 10 Jul, 2008, 11:15: AM
Concept Videos
CBSE 12-science - Physics
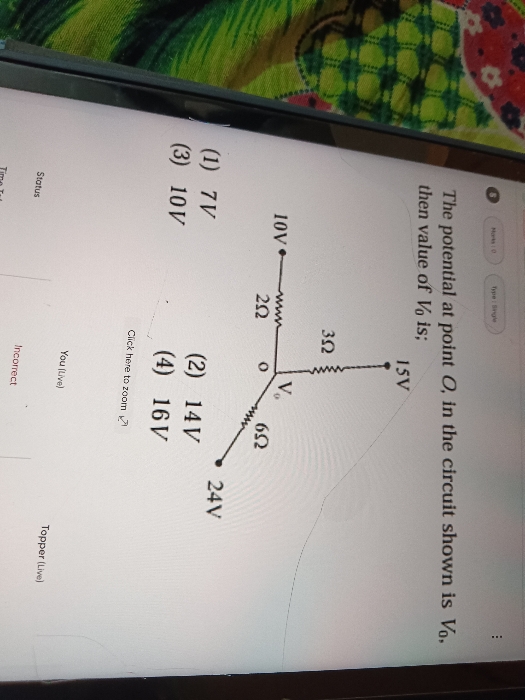
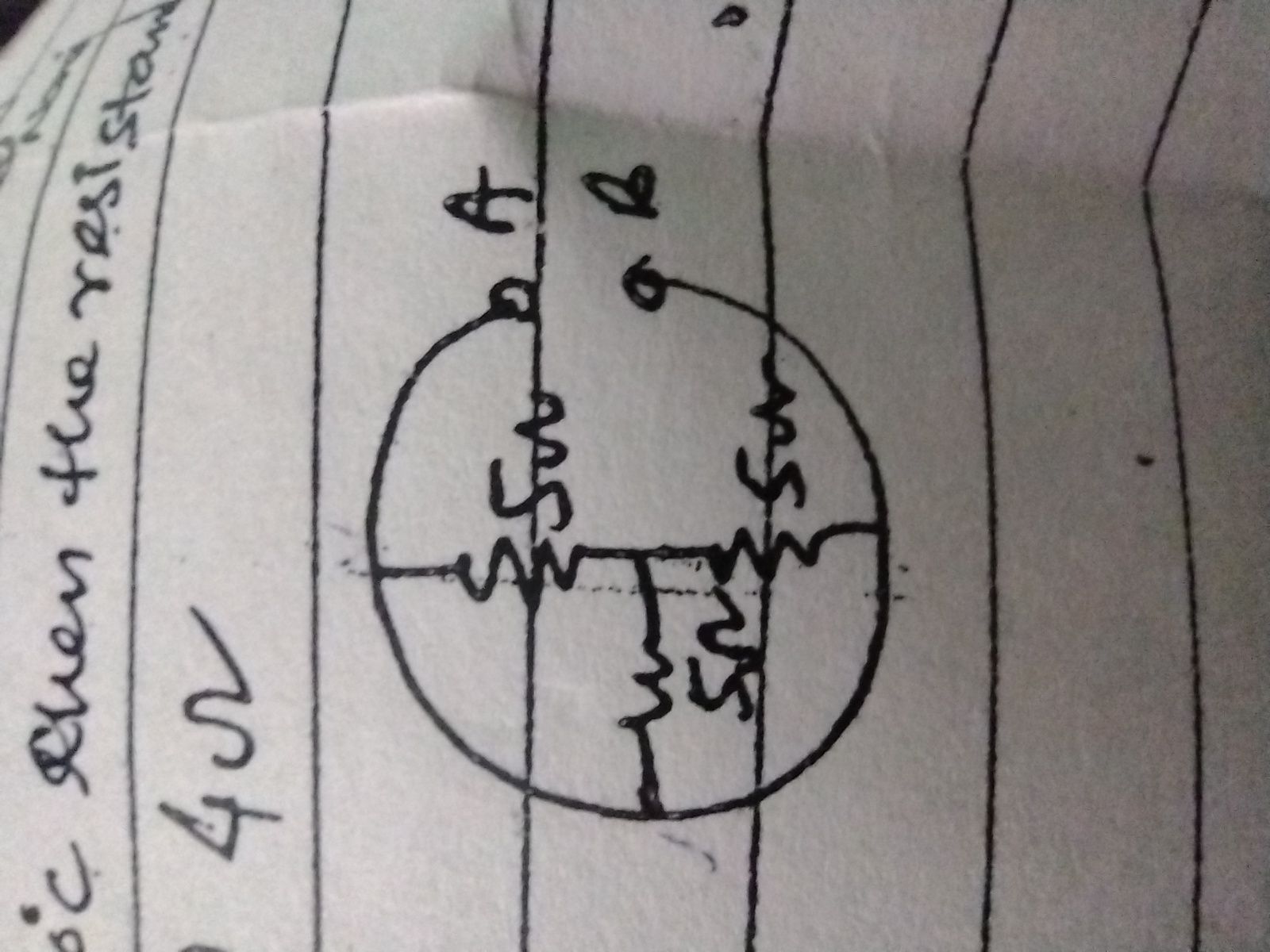
Asked by mailtoanjalip2005 | 16 Mar, 2024, 08:22: PM
CBSE 12-science - Physics
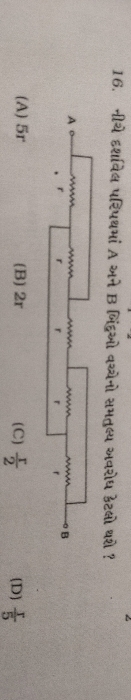
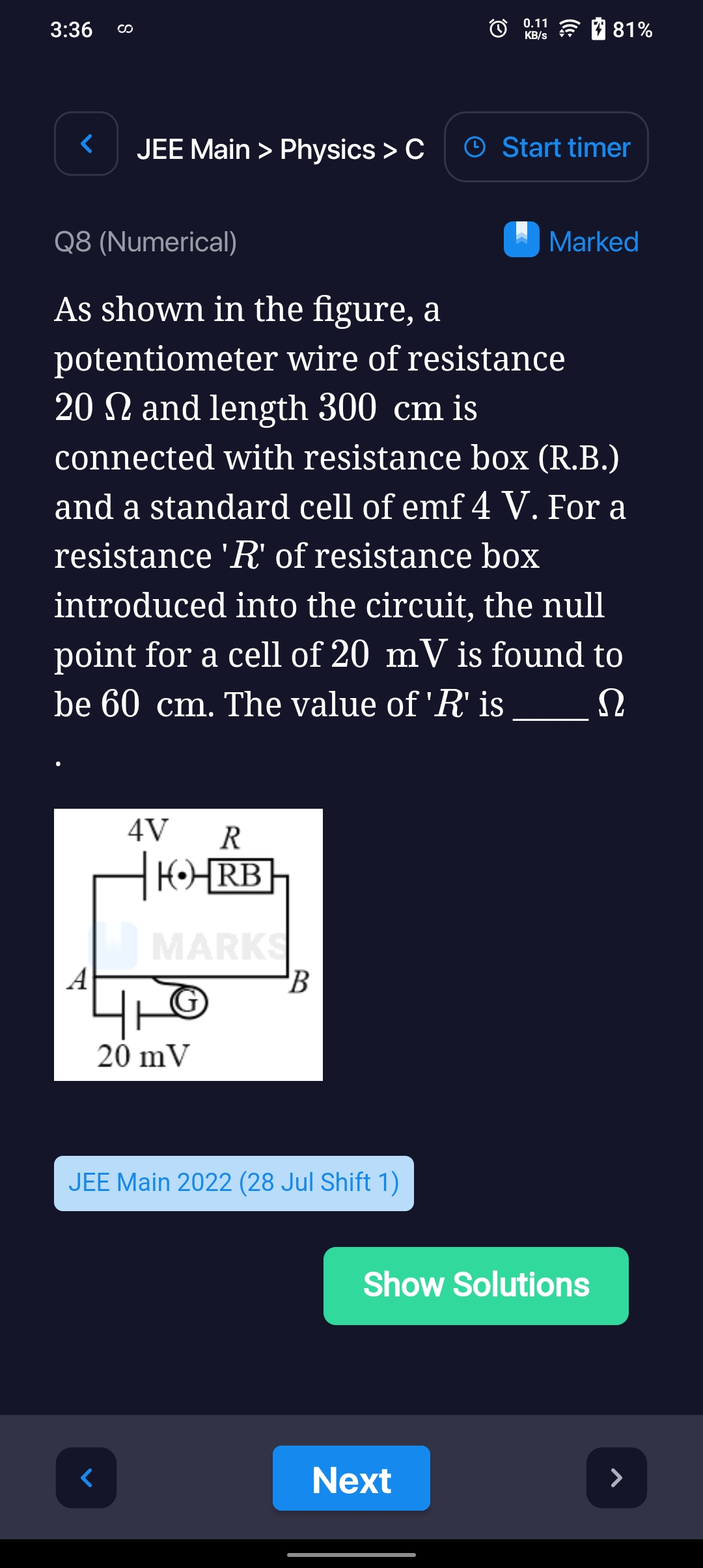
Asked by patelnamra608 | 26 Jan, 2024, 11:01: AM
CBSE 12-science - Physics
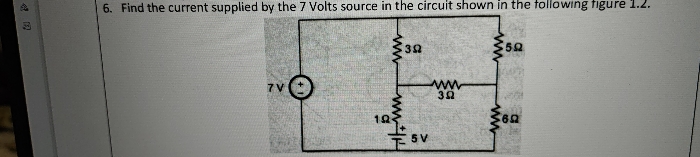
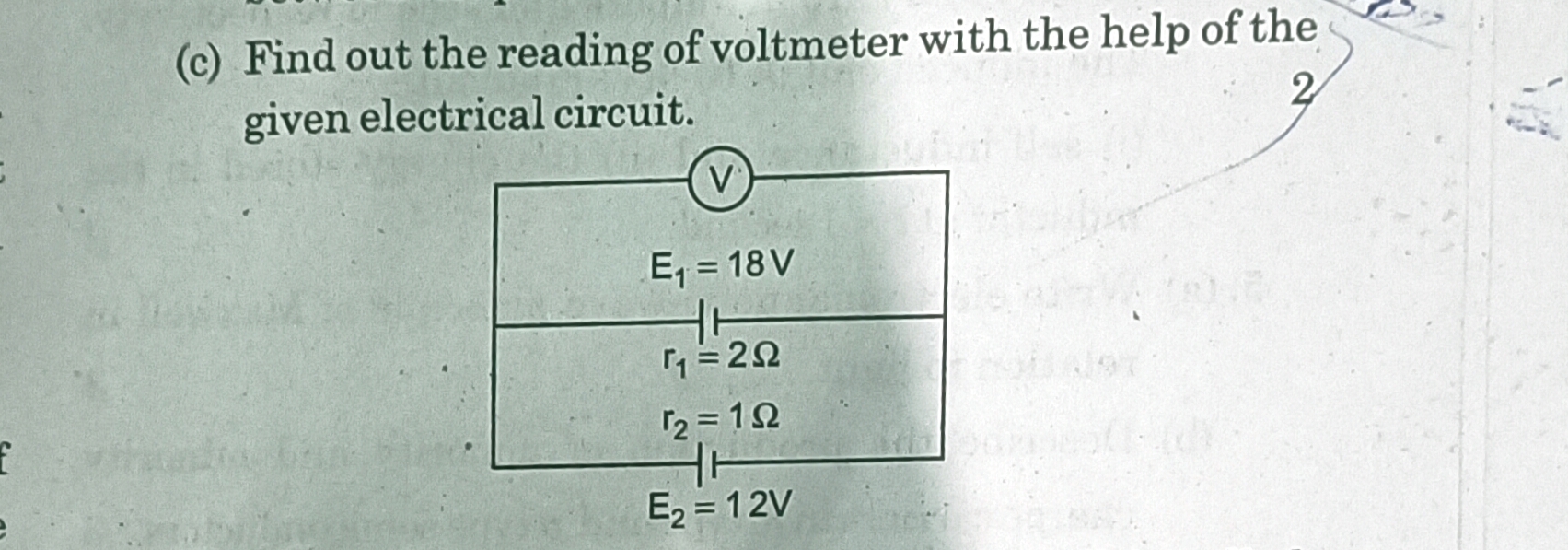
Asked by smitdholakiya28 | 17 Dec, 2023, 09:37: AM
CBSE 12-science - Physics
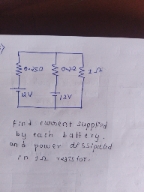
Asked by shivashikhar69 | 09 Dec, 2023, 02:27: PM
CBSE 12-science - Physics
Asked by nikhilsai2616 | 19 Nov, 2023, 01:05: AM
CBSE 12-science - Physics
Asked by snehashiragannavar773 | 21 Oct, 2023, 02:38: PM
CBSE 12-science - Physics
Asked by dr.strange45678 | 02 Aug, 2023, 12:31: PM
CBSE 12-science - Physics
Asked by komalbrar0987 | 17 May, 2023, 06:15: PM
CBSE 12-science - Physics
Asked by amanpanday6384 | 22 Dec, 2022, 12:42: PM