CBSE Class 12-science Answered
Explain the THEORY OF HETEROGENOUS CATALYSIS in detail with the help of examples
Asked by pardeepkumar2281 | 31 Jul, 2018, 11:38: PM
Heterogeneous Catalysis:
If the catalyst is present in the different phase than that of the reactants, it is called a heterogeneous catalyst and
this type of catalysis is called heterogeneous catalysis.
- The catalyst in heterogeneous catalysis, catalyst is in solid and the reactants are gases or liquids.
- Also, the reaction takes place at the surface of the solid catalyst hence it is called as surface catalysis.
- According to old adsorption theory, it was assumed that the reactants in the gaseous state or from the solutions are adsorbed on the surface of the catalyst.
- Because of this, the concentration of the reactant molecules on the surface increases and the rate of reaction also increases.
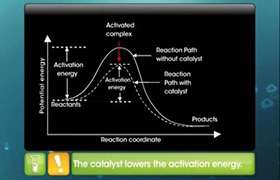
- Adsorption is exothermic hence the heat evolved during the reaction is utilized to speed up the reaction.
Example:
Manufacture of ammonia from N2 and H2 by Haber’s process.

Answered by Varsha | 01 Aug, 2018, 10:19: AM
Concept Videos
CBSE 12-science - Chemistry
Asked by sivakapuganti1 | 26 Aug, 2020, 08:57: PM
CBSE 12-science - Chemistry
Asked by spoorthysaienelluri | 23 May, 2020, 11:07: AM
CBSE 12-science - Chemistry
Asked by vermahitesh124 | 12 May, 2020, 07:58: AM
CBSE 12-science - Chemistry
Asked by yogeshsulakh | 07 Feb, 2020, 09:28: AM
CBSE 12-science - Chemistry
Asked by tlb2bpartner | 21 Aug, 2019, 10:28: PM
CBSE 12-science - Chemistry
Asked by niharikapabba2605 | 07 Aug, 2018, 11:38: AM
CBSE 12-science - Chemistry
Asked by pardeepkumar2281 | 31 Jul, 2018, 11:40: PM
CBSE 12-science - Chemistry
Asked by pardeepkumar2281 | 31 Jul, 2018, 11:38: PM
CBSE 12-science - Chemistry
Asked by pardeepkumar2281 | 31 Jul, 2018, 11:36: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM