CBSE Class 10 Answered
Explain the Newland's Law of Octaves.
Asked by pdcavita | 24 Nov, 2017, 08:52: AM
Newlands’ Law of Octaves for 2 marks
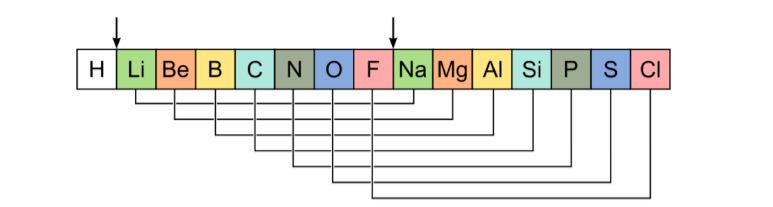
- In 1864, Newlands arranged the known 56 elements in the order of increasing atomic masses.
- He observed that the properties of every eighth element are similar to the properties of the first element.
- Based on this observation, he proposed the Law of Octaves for the classification of elements.
- Law of Octaves: When the elements are arranged in the increasing order of their atomic masses, the properties of every eighth element are similar to the first.
Answered by Ramandeep | 24 Nov, 2017, 10:02: AM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by kavithasenthil148 | 06 Feb, 2021, 07:09: PM
CBSE 10 - Chemistry
Asked by mina6poonam | 21 Jan, 2021, 04:57: PM
CBSE 10 - Chemistry
Asked by dudalaganesh | 04 May, 2020, 08:35: AM
CBSE 10 - Chemistry
Asked by abhisv8953 | 21 Apr, 2020, 02:59: PM
CBSE 10 - Chemistry
Asked by bikashlakhya2003 | 23 Jan, 2020, 07:40: PM
CBSE 10 - Chemistry
Asked by webfaridisit | 06 Jan, 2020, 08:56: PM
CBSE 10 - Chemistry
Asked by vermamahima021982 | 27 Aug, 2019, 09:50: PM
CBSE 10 - Chemistry
Asked by saradamahapatra9999 | 10 Aug, 2019, 11:35: AM
CBSE 10 - Chemistry
Asked by prathameshmane3622 | 10 Jul, 2019, 08:52: PM
CBSE 10 - Chemistry
Asked by 123cmb123 | 12 Dec, 2018, 10:02: PM









