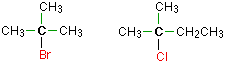CBSE Class 12-science Answered
Haloalkanes or Halogenoalkanes are compounds in which one or more hydrogen atoms of an alkane have been replaced by halogen atoms (fluorine, chlorine, bromine or iodine).
Few examples:
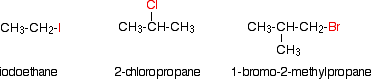
Depending upon the position of the halogen atoms, the haloalkanes are classified as primary,secondary and tertiary.
In a primary (1°) halogenoalkane, the carbon which carries the halogen atom is only attached to one other alkyl group.
![]()
In a secondary (2°) halogenoalkane, the carbon with the halogen attached is joined directly to two other alkyl groups, which may be the same or different.
Examples:
![]()
In a tertiary (3°) halogenoalkane, the carbon atom holding the halogen is attached directly to three alkyl groups, which may be any combination of same or different.