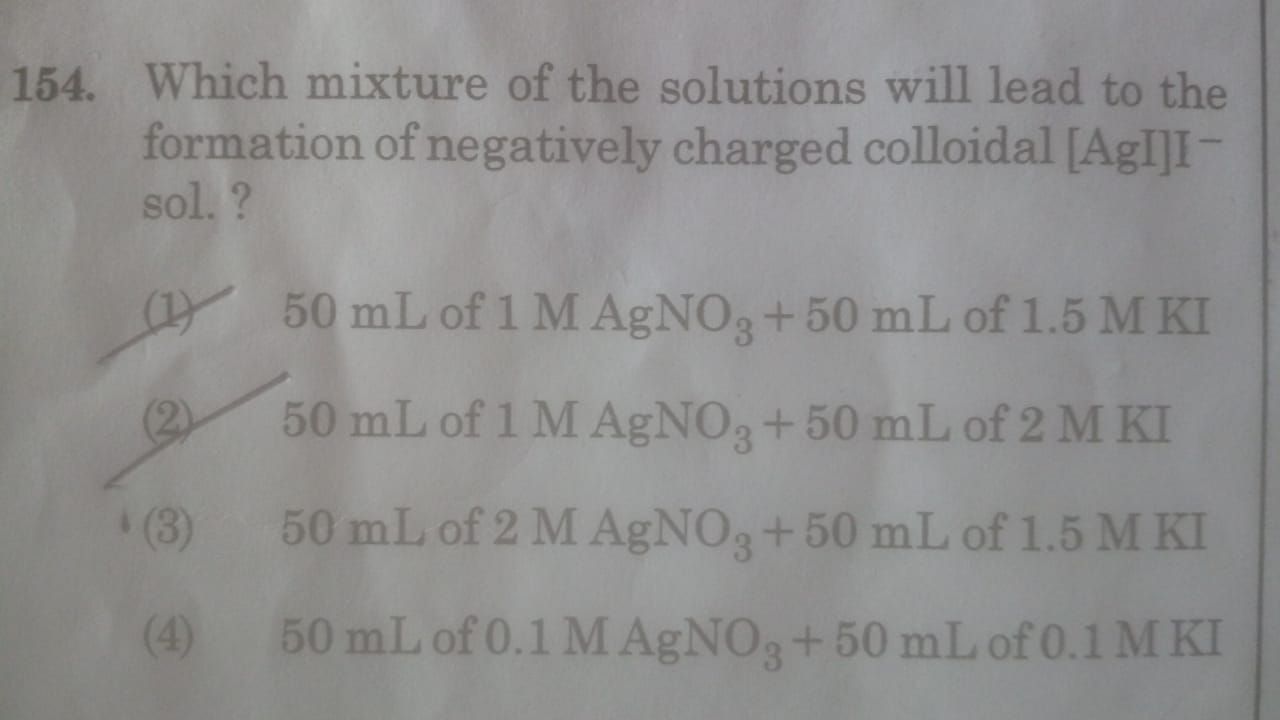CBSE Class 12-science Answered
Explain the following Observation
Fe(OH)3 SOL COAGULATES ON ADDITION OF A SOLUTION OF K2 SO4
Asked by vaibhav gupta | 30 Jun, 2013, 03:15: PM
When an electrolyte is added to the sol, the colloidal particles take up ions carrying opposite charges from the electrolyte. As a result, their charge gets neutralised and this causes the uncharged particles to come closer and to get coagulated. Here Fe3+ ions of ferric hydroxide are attracted by the negatively charged SO42- sol particles and their charge gets neutralised. This leads to coagulation.
Answered by | 01 Jul, 2013, 10:20: AM
Application Videos
Concept Videos
CBSE 12-science - Chemistry
Asked by mallavaramchandrika6 | 29 Sep, 2021, 05:27: PM
CBSE 12-science - Chemistry
Asked by Akshij Nanda | 11 Mar, 2021, 10:21: AM
CBSE 12-science - Chemistry
Asked by sivakapuganti1 | 26 Aug, 2020, 08:57: PM
CBSE 12-science - Chemistry
Asked by spoorthysaienelluri | 23 May, 2020, 11:07: AM
CBSE 12-science - Chemistry
Asked by shreemuvijihari | 18 May, 2020, 06:29: PM
CBSE 12-science - Chemistry
Asked by vermahitesh124 | 12 May, 2020, 07:58: AM
CBSE 12-science - Chemistry
Asked by yogeshsulakh | 07 Feb, 2020, 09:28: AM
CBSE 12-science - Chemistry
Asked by tlb2bpartner | 21 Aug, 2019, 10:28: PM
CBSE 12-science - Chemistry
Asked by ntg432000 | 22 May, 2019, 08:10: PM
CBSE 12-science - Chemistry
Asked by kripanjalihimansu | 01 Mar, 2019, 04:16: PM