CBSE Class 11-science Answered
Explain the difference between wetting agents and water-proof agents with respect to the phenomenon of surface tension.
Asked by MAANASI.M | 18 Nov, 2013, 07:11: AM
A substance that reduces the surface tension of a liquid, causing the liquid to spread across surface of a solid.
If such substances are added to the liquid, it lowers the surface tension and liquid can easily spread on the surface.
Contact angle is the angle a drop water makes with solid surface.
If contact angle is low liquid drop can easilly spread. Ex: water can easily wet the surface of a cottan cloth. Detergent also one example of wetting agent.
Water proof agents increases the surface tention hence the contact angle. Thus liquid can not spread on the surface. Ex: water beads on a wax or oily surface. water can not wet the oily surface because of high contact angle.
Answered by Komal Parmar | 19 Nov, 2013, 03:21: PM
Concept Videos
CBSE 11-science - Physics
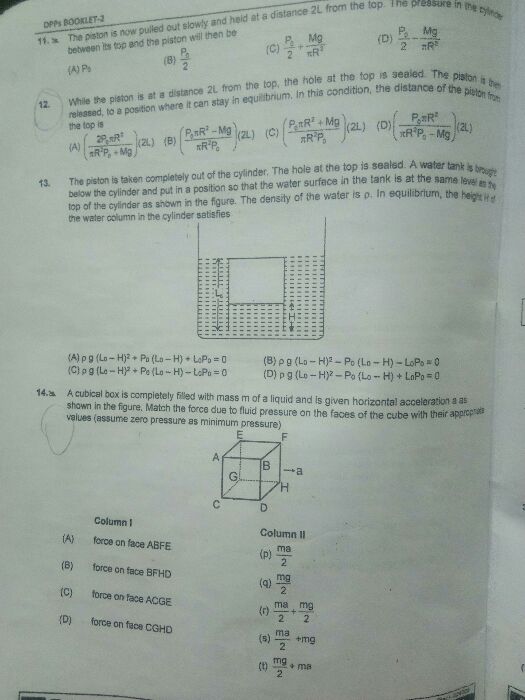
Asked by rajuinwati12 | 04 Mar, 2024, 09:22: AM
CBSE 11-science - Physics
Asked by joelisaacsam | 09 Jan, 2024, 06:31: AM
CBSE 11-science - Physics
Asked by rupimadhes | 21 Nov, 2023, 05:31: AM
CBSE 11-science - Physics
Asked by arjunsah797 | 12 Mar, 2022, 11:10: AM
CBSE 11-science - Physics
Asked by suragimathpraveen2001 | 10 Jan, 2022, 08:06: PM
CBSE 11-science - Physics
Asked by raghuvanshisaumya461 | 03 Dec, 2021, 08:05: AM
CBSE 11-science - Physics
Asked by abhinavakrishna1009 | 31 Mar, 2021, 11:15: AM
CBSE 11-science - Physics
Asked by vanshraaj.ind | 13 Dec, 2020, 06:06: PM
CBSE 11-science - Physics
Asked by ashanihalchandani | 26 May, 2020, 09:11: AM
CBSE 11-science - Physics
Asked by mahimanaik290 | 10 May, 2020, 12:45: AM