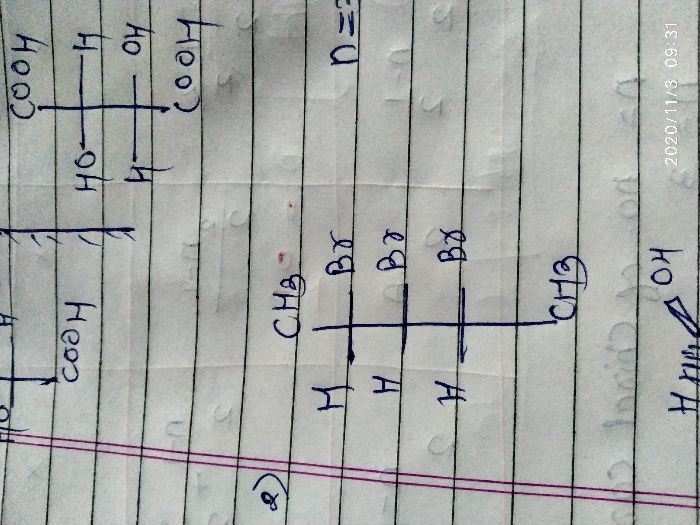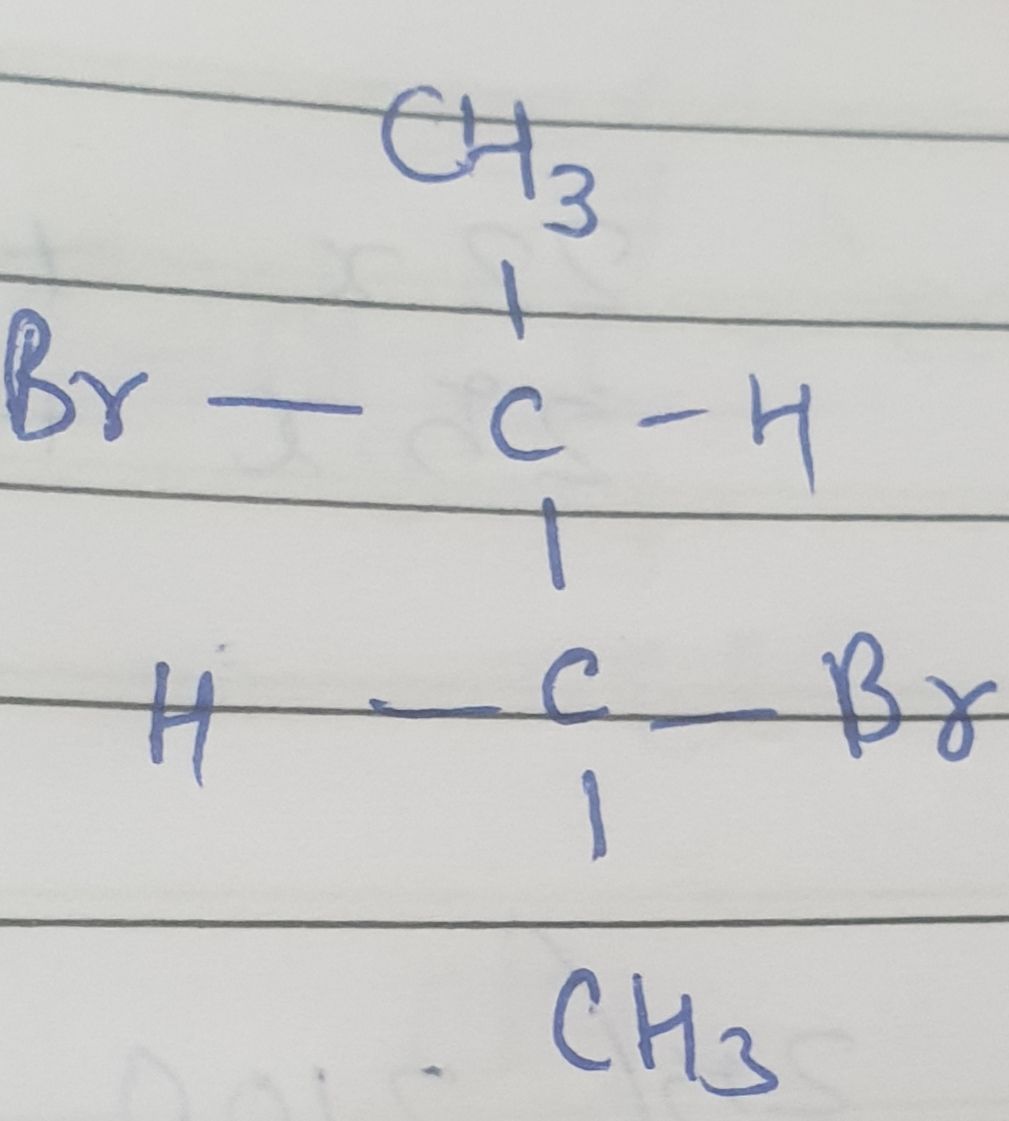CBSE Class 11-science Answered
Stereoisomerism are of two types:
1) Conformational isomerism
2) Configurational isomerism
Configurational isomerism is further classified as:
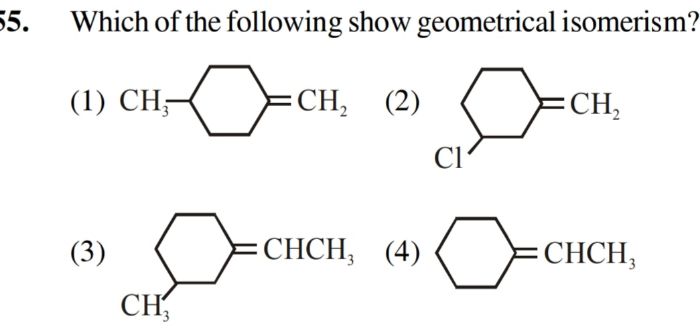
1) Geometrical isomerism
2) Optical isomerism
Optical isomers and optical isomerism:
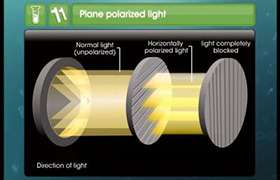
‘Optical isomers’ name is given to the compounds because of their effect on the plane polarized light. Optical isomers are the two compounds which are non-superimposable mirror images of each other and rotate the plane of plane polarized light in opposite direction to each other. The phenomenon shown by optical isomers is known as ‘optical isomerism’.
Optical isomers are chiral molecules. Chiral molecules are non-superimposable mirror images of each other due to the presence of asymmetric carbon atom.
A carbon atom attached to four different substituents (atoms or groups) is called an asymmetric or chiral carbon atom. Chiral atom is indicated by an asterisk (*).
There are four elements of symmetries such as plane of symmetry, centre of symmetry, alternating axis of symmetry and simple axis of symmetry. Chiral molecules do not have any element of symmetry.
Optical isomerism in compounds having one chiral centre:
Optical isomers which are non-superimposable mirror images of each other are called enantiomers and the phenomenon is called enantiomerism. The enantiomers have identical physical and chemical properties but rotate the plane of polarized light in opposite directions but to the same extent.
Enantiomer which can rotate the plane polarized light in right direction is called dextrorotatory and the other one which rotate plane polarized light in left direction is called laevorotatory.
For example, Lactic acid