CBSE Class 12-science Answered
Explain:
(i)The treatment of alkyl chlorides with aqKOH leads to the formation of alcohols,but in the presence of alcoholic KOH,alkenes are formed.
(ii)Out of C6H5CH2Cl and C6H5ClC6H5 which is more easily hydrolysed by aqKOH and why?
Asked by banga71 | 13 Jan, 2018, 11:32: AM
(1) Alkyl chloride in presence of aqueous KOH gives the substituted product. In an aqueous solution, KOH completely ionizes to give hydroxide ions. OH- ion is a strong nucleophile, which can easily substitute the strong leaving chloride ion and leads to the formation of alcohol.
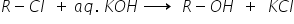
On other hand alkyl chloride containing β-hydrogen atom with alcoholic KOH solution undergo elimination reaction to give alkene as a major products. An alcoholic solution of KOH produce alkoxide (RO- ) ion, which is a strong base. It can easily abstract a β-carbon of alkyl chloride and form an alkene as a major product.

(2)
Answered by Ramandeep | 15 Jan, 2018, 09:42: AM
Application Videos
Concept Videos
CBSE 12-science - Chemistry
Asked by surajbhanupatro44 | 07 Nov, 2023, 12:01: AM
CBSE 12-science - Chemistry
Asked by mayamishra9540500880 | 04 Jul, 2022, 07:11: PM
CBSE 12-science - Chemistry
Asked by harshaldpathak | 11 Jun, 2022, 05:37: PM
CBSE 12-science - Chemistry
Asked by amitkumar.cis | 01 Jan, 2021, 09:15: PM
CBSE 12-science - Chemistry
Asked by me.mirzainayat | 14 Nov, 2020, 07:31: AM
CBSE 12-science - Chemistry
Asked by Prachidewangan74 | 02 Oct, 2020, 03:02: PM
CBSE 12-science - Chemistry
Asked by sujithanathan119 | 01 Jun, 2020, 12:00: PM
CBSE 12-science - Chemistry
Asked by ng9045007209 | 21 May, 2020, 07:47: PM
CBSE 12-science - Chemistry
Asked by gangavaramouni | 26 Mar, 2020, 10:33: AM