CBSE Class 11-science Answered
explain hybridisation and molecular orbital theory in short...............
Asked by Joepeter | 13 Sep, 2014, 09:02: PM
Dear jose4.peter42@gmail.com
Thanks for asking us a question in Ask the Expert section of TopperLearning.com.
In case of multiple questions within a query, please post each question individually and let us know where you are getting stuck so that we would be able to explain things better.
Solution of your first query:
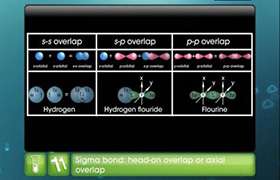
Hybridisaton can be defined as the mixing of atomic orbitals belonging to the same atom but have slightly different energies. On mixing same number of new hybrid orbitals of same energies are formed.
For example:
sp hybrid orbitals: when one s and one p orbital get mixed to form two sp-orbital with 180o bond angle.
Regards
Topperlearning Team.
Topperlearning Team.
Answered by Prachi Sawant | 15 Sep, 2014, 09:31: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by Trisha Gupta | 30 Oct, 2022, 05:36: PM
CBSE 11-science - Chemistry
Asked by ABHILASHA | 22 Aug, 2020, 04:39: AM
CBSE 11-science - Chemistry
Asked by kpbhake | 12 Mar, 2018, 11:45: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 08 Oct, 2014, 01:09: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 13 Jun, 2016, 02:26: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 11-science - Chemistry
What is the hybrid state of B in BF3, Al in AlCl3, Be in BeCl2, C in CO2 and C2H4; S in SO2 and SO3.
Asked by Topperlearning User | 08 Oct, 2014, 01:33: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 09 Oct, 2014, 09:30: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM


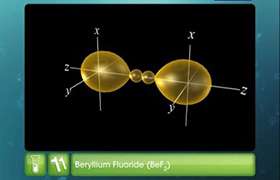
 on the basis of hybridisation
on the basis of hybridisation