CBSE Class 12-science Answered
explain how does the -oh group attached to carbon of benzene ring activate it towards electrophilic substitution?
Asked by ATUL raizada | 23 Aug, 2012, 10:24: PM
The -OH group shows +M effect due to delocalization of lone pair on oxygen atom towards the ring. Thus the electron density on benzene ring is increased particularly on ortho and para positions.
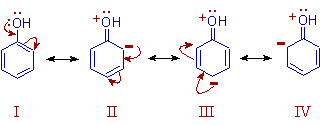
Hence phenol is more reactive towards electrophilic substitution reactions. The substitution is favored more at ortho and para positions.
Answered by | 24 Aug, 2012, 06:12: PM
Application Videos
Concept Videos
CBSE 12-science - Chemistry
Asked by kavitabawane190 | 08 Mar, 2024, 05:24: PM
CBSE 12-science - Chemistry
Asked by rp0055293 | 07 Feb, 2024, 08:28: AM
CBSE 12-science - Chemistry
Asked by sanyaagrahari8888 | 28 Nov, 2023, 01:07: PM
CBSE 12-science - Chemistry
Asked by bithikapurkait09052005 | 24 Jun, 2022, 08:35: AM
CBSE 12-science - Chemistry
Asked by rohanm | 14 Feb, 2022, 04:49: PM
CBSE 12-science - Chemistry
Asked by vipulverma | 14 Feb, 2022, 04:44: PM
CBSE 12-science - Chemistry
Asked by kaziryan.05 | 23 Jun, 2021, 08:02: PM
CBSE 12-science - Chemistry
Asked by deepakatur454 | 28 Nov, 2020, 04:29: PM
CBSE 12-science - Chemistry
Asked by holebasubudihal | 09 Aug, 2020, 02:58: PM
CBSE 12-science - Chemistry
Asked by sulaikhasulu393 | 12 Jun, 2020, 09:34: PM