CBSE Class 10 Answered
Explain Carbon Atomic structure
Asked by akshay.beloshe | 04 Jul, 2018, 08:06: PM
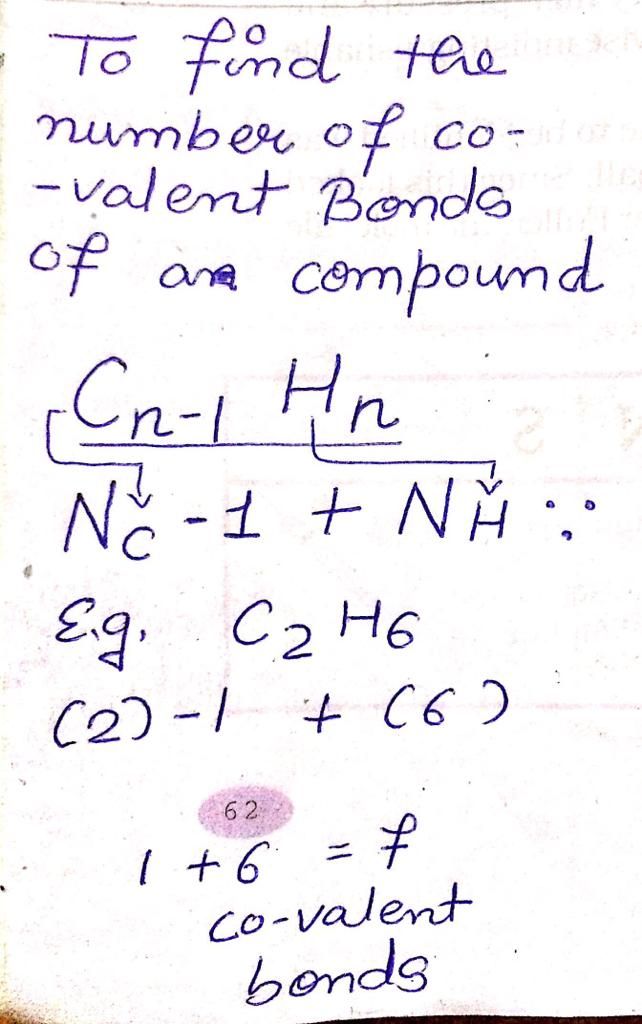
Atomic structure of carbon:
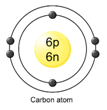
Therefore,
Atomic number [Z] = no. of protons [p] = no. of electrons [e]
Z = 6 = 6
Therefore, the atomic number of carbon is 6.
And Atomic mass number [A] = no. of protons [p] + no. of netrons [n]
A = 6 + 6
A = 12
Therefore, the atomic mass number of carbon is 12.
Answered by Ramandeep | 06 Jul, 2018, 11:31: AM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by tialempongen177 | 09 Sep, 2020, 11:44: PM
CBSE 10 - Chemistry
Asked by seeni2005 | 05 Jul, 2020, 09:53: PM
CBSE 10 - Chemistry
Asked by subbukum | 04 Feb, 2020, 11:46: AM
CBSE 10 - Chemistry
Asked by navjotsinghdadwal | 01 Dec, 2019, 09:57: PM
CBSE 10 - Chemistry
Asked by 9886761796hmh | 17 Oct, 2019, 08:10: PM
CBSE 10 - Chemistry
Asked by ashishaman25082004 | 15 Sep, 2019, 10:01: PM
CBSE 10 - Chemistry
Asked by dr.sudhiguptajdmd74 | 23 Jul, 2019, 09:18: AM
CBSE 10 - Chemistry
Asked by labheshvaidya | 19 Apr, 2019, 03:38: PM
CBSE 10 - Chemistry
Asked by krishdabhoya2003 | 21 Feb, 2019, 05:52: PM
CBSE 10 - Chemistry
Asked by amitpass78 | 09 Jan, 2019, 10:35: PM