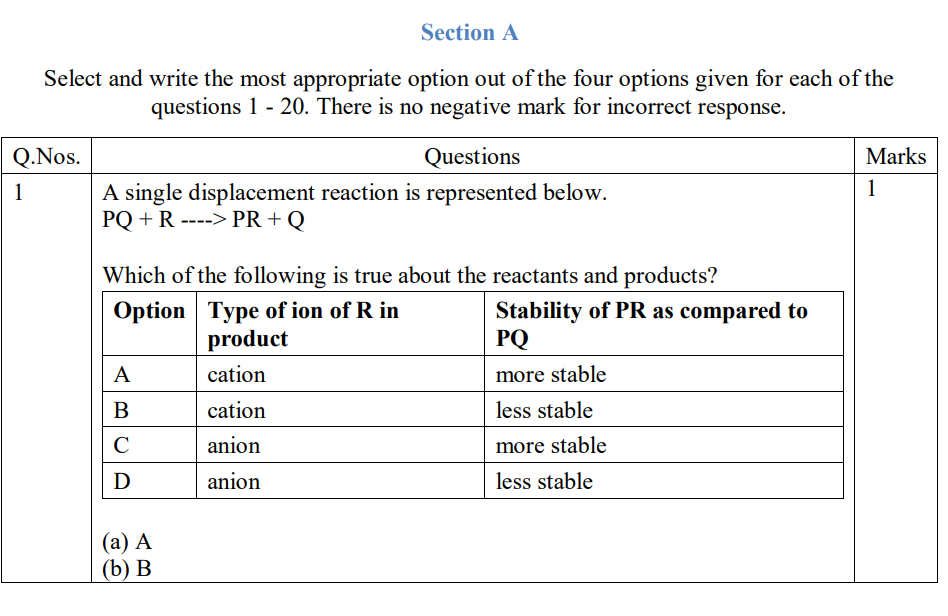
CBSE Class 10 Answered
A chemical reaction in which two or more substances combine to form a single product is called a combination reaction or synthesis.
When iron and sulphur are heated together, they combine to form a single product, iron sulphide.
2Fe(s) + S(s) → FeS (s)
Iron Sulphur Iron sulphide
2. Decomposition Reaction :
A chemical reaction in which a single compound splits into two or more simple substances is called a decomposition reaction.
When mercuric oxide is heated in a crucible, the orange-red powder begins to darken and a silver mirror begins to deposit on the cooler parts of the crucible. This is mercury. If we hold a glowing splint near the crucible, it can relight. This shows that the gas evolved during the reaction is oxygen.
2HgO(s) 2Hg(s) + O2 ↑
Mercuric oxide Mercury Oxygen
3.Displacement Reaction:
Reactions in which the more reactive element displaces the less reactive element from its compound are called displacement reactions.
Zinc displaces copper in copper sulphate to form zinc sulphate.
Zn(s) + CuSO4(aq) → ZnSO4(aq) + Cu(s)
Zinc Copper sulphate Zinc sulphate4.Double Displacement Reaction:
A type of chemical change in which two compounds in a solution react to form two new compounds by the mutual exchange of radicals. Usually, a solid is formed as a result of the reaction.
NaOH(aq) + HCl(aq) → NaCl(aq) + H2O(l)
Sodium Hydrochloric Sodium Water
hydroxide acid chloride
5. Redox Reaction:
The oxidation and reduction reactions occur together. When oxidation of one substance occurs, reduction of the other substance also takes place. Such reactions are called redox reactions. In the name ‘redox’, the term ’red’stands for reduction and ’ox’ stands for oxidation.
Consider the redox reaction between hydrogen sulphide and chlorine.
In the reaction between hydrogen sulphide and chlorine, the products formed are hydrogen chloride and sulphur.
H2S + Cl2 → 2HCl + S
Hydrogen Chlorine Hydrogen Sulphur