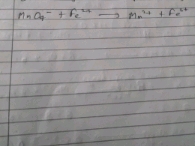CBSE Class 11-science Answered
one equivalent (or equivalent weight) of a substance is the amount of that substance which supplies or consumes one mol of reactive species. In acid-base chemistry the reactive species is the hydrogen ion (H1+) while in oxidation-reduction chemistry the reactive species is the electron. For example, in the following two reactions the equivalent weight of H2SO4 would be 49 grams or 0.5 mol in the first reaction but 98 grams or 1 mol in the second. On the other hand, sodium hydroxide has the same equivalent weight in both reactions, one mol or 40 grams.
(1) H2SO4 + 2NaOH ------> Na2SO4 + 2H2O
(2) H2SO4 + NaOH ------> NaHSO4 + H2O
In the first reaction one mol of H2SO4 supplies 2 mols of H1+ to NaOH, therefore, one-half mol of H2SO4 or 49 grams is one equivalent. The conditions are different in the second reaction because sulfuric acid only "looses" one hydrogen so the equivalent weight of sulfuric acid is one mol or 98 grams. However, sodium hydroxide behaves the same in both reactions, that is, one mol of sodium hydroxide always "consumes" one mol of H1+, so its equivalent weight remains the same at one mol or 40 grams.
In order to determine the equivalent weight of a substance you must know something about the reaction but it does not have to be balanced. Equivalents can help in the analysis of a substance when the balanced reaction is not known or cannot be written for whatever reason; because one equivalent always reacts with one equivalent. (You should prove this to yourself by calculating how much sodium hydroxide is needed to react with 49 grams of sulfuric acid in each of the two reactions above. Do your calculations using traditional mol relationships and the one to one relationship for equivalents.)