CBSE Class 11-science Answered
Eletronegativity:- what will be defination in case an isolated atom? There is no bonding pair of electrons.
Asked by Asamanja | 26 Jun, 2014, 08:35: PM
Electronegativity is a property of an atom which is fixed for that atom whether it is in isolated state or combined state with some other atom. Hence, electronegativity can be defined as the tendency of an isolated atom to attract bonding pair of electrons.
Answered by Prachi Sawant | 27 Jun, 2014, 10:47: AM
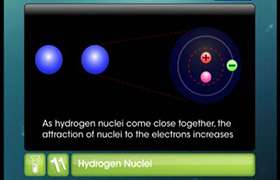
Concept Videos
CBSE 11-science - Chemistry
Asked by visalvinod06 | 23 Jun, 2022, 07:39: AM
CBSE 11-science - Chemistry
Asked by bhagwatkrutika6 | 22 Jun, 2022, 09:53: PM
CBSE 11-science - Chemistry
Asked by naveenbahuguna05 | 11 Dec, 2021, 03:19: PM
CBSE 11-science - Chemistry
Asked by akhileshpandeypandey456 | 12 Aug, 2021, 11:09: PM
CBSE 11-science - Chemistry
Asked by toshan.dandi5 | 24 Feb, 2021, 09:24: PM
CBSE 11-science - Chemistry
Asked by patilanil3766 | 01 Jan, 2021, 10:28: PM
CBSE 11-science - Chemistry
Asked by anjalikri2201 | 08 Aug, 2020, 11:22: AM
CBSE 11-science - Chemistry
Asked by khushipatel2124 | 07 Jul, 2020, 04:23: PM
CBSE 11-science - Chemistry
Asked by saurabhchaddha4205 | 03 Jun, 2020, 04:22: PM
CBSE 11-science - Chemistry
Asked by utkarshnikam85.tl | 06 Apr, 2020, 12:13: PM