ICSE Class 10 Answered
Element X has 2 valence electrons ,element X has atomic number 7.
A) Write equation to show how X and Y form ions.
B) If B is a diatomic gas ,write the equation for the direct combination of X and Y to form a compound.
Asked by mittaljeevesh | 11 Dec, 2018, 07:06: PM
Element X has 2 valence electrons which means it has valency = 2
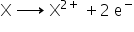
element X has atomic number 7. Its electronic arrangement is (2,5) which means it's valency is 3.
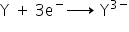
The equation for the direct combination of X and Y to form a compound.
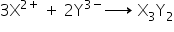
Answered by Ramandeep | 12 Dec, 2018, 11:03: AM
Application Videos
Concept Videos
ICSE 10 - Chemistry
Asked by navedsheikh97658 | 01 Nov, 2023, 04:57: PM
ICSE 10 - Chemistry
Asked by adityagogineni15.10spicertl | 18 Jun, 2020, 02:01: PM
ICSE 10 - Chemistry
Asked by dhruvasavaliya7916.10sdatl | 24 Apr, 2020, 09:48: PM
ICSE 10 - Chemistry
Asked by rohitmodi785 | 10 Dec, 2019, 09:28: AM
ICSE 10 - Chemistry
Asked by Babyka111 | 03 Jun, 2019, 03:24: PM
ICSE 10 - Chemistry
Asked by mittaljeevesh | 11 Dec, 2018, 07:06: PM
ICSE 10 - Chemistry
Asked by alshivakumar12 | 03 Jul, 2018, 06:11: PM
ICSE 10 - Chemistry
Asked by Nikhil | 12 Jun, 2018, 07:21: AM
ICSE 10 - Chemistry
Asked by 21janhvi.verma | 06 Jun, 2018, 10:29: AM
ICSE 10 - Chemistry
Asked by Shreya | 13 Mar, 2018, 10:05: AM






