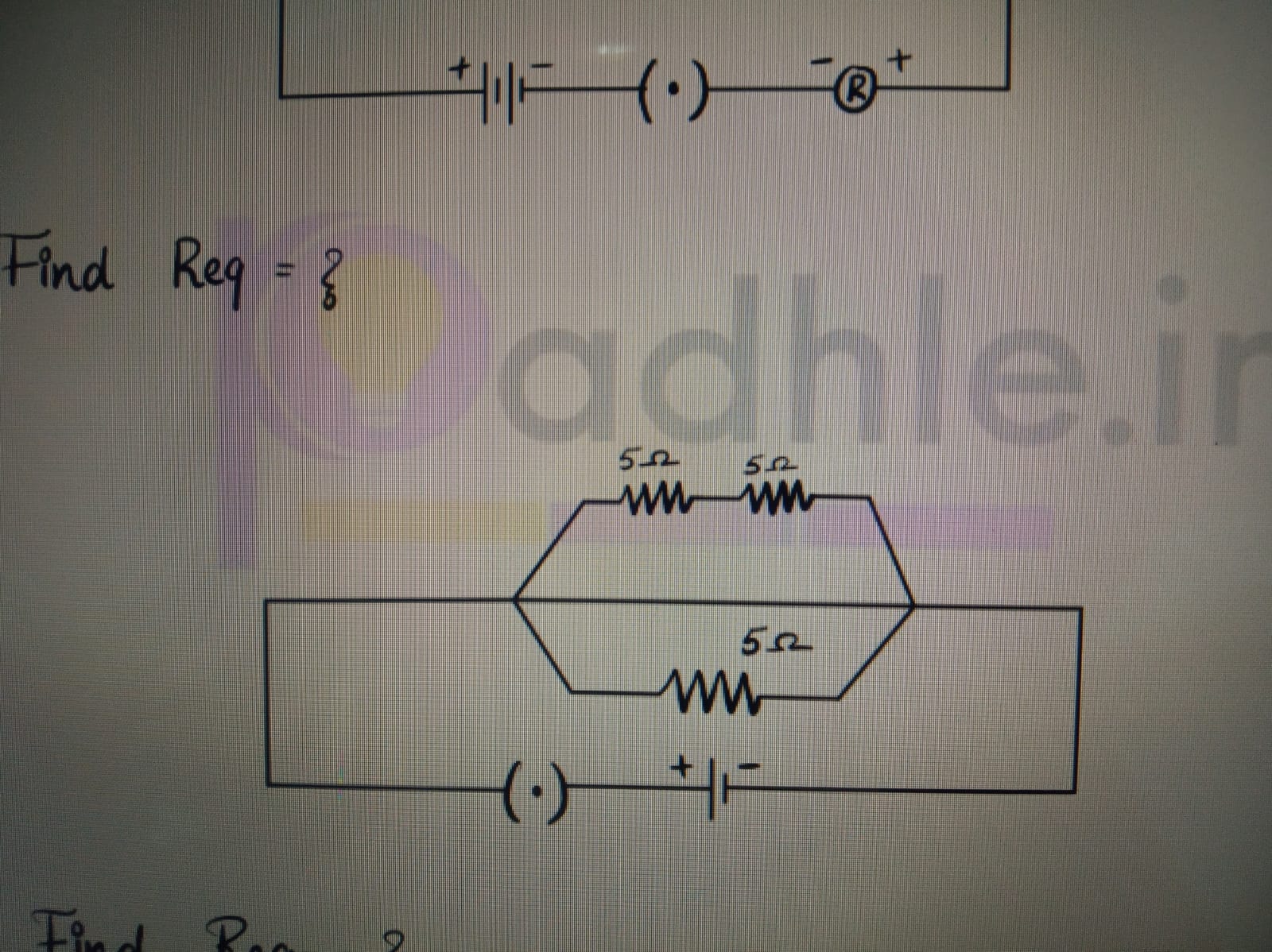
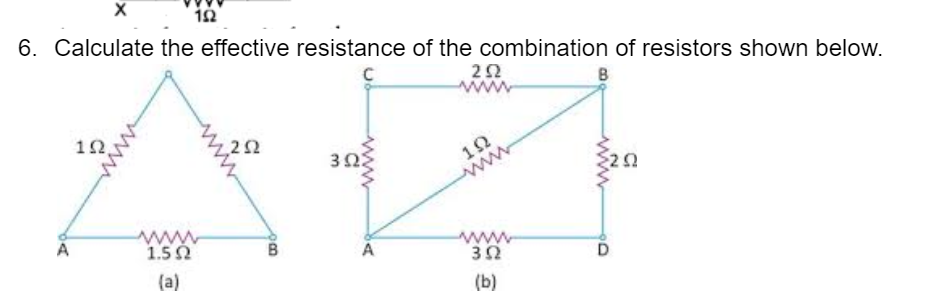
CBSE Class 10 Answered
Dry NaCL not conduct electricity but aqueous NaCL does conduct electricity why?
Asked by spuneet23 | 18 Jan, 2010, 05:44: PM
Dry NaCl have intact crystal structure, no free ions to conduct electricity, but in aqueous, NaCl dissociates into Na+ and Cl- ions which conduct electricity.
Regards,
Team,
TopperLearning.
Answered by | 18 Jan, 2010, 07:56: PM
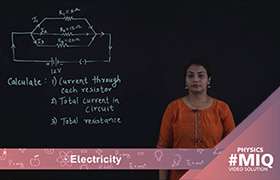
Application Videos
Concept Videos
CBSE 10 - Physics
Asked by khajannirwan | 27 Feb, 2024, 10:20: PM
CBSE 10 - Physics
Asked by saanviyadla | 24 Jan, 2024, 07:06: PM
CBSE 10 - Physics
Asked by kamalaranjanmohantymohanty5 | 06 Jan, 2024, 10:05: AM
CBSE 10 - Physics
Asked by nandhikasugumar | 05 Oct, 2023, 04:01: PM
CBSE 10 - Physics
Asked by daniya062008 | 02 Oct, 2023, 08:25: PM
CBSE 10 - Physics
Asked by prassanna.j | 03 Sep, 2023, 12:28: PM
CBSE 10 - Physics
Asked by prassanna.j | 03 Sep, 2023, 12:21: PM
CBSE 10 - Physics
Asked by prassanna.j | 03 Sep, 2023, 12:13: PM
CBSE 10 - Physics
Asked by prassanna.j | 03 Sep, 2023, 12:11: PM