CBSE Class 11-science Answered
Does surface tension act tangentially to the surface of liquid or towards the interior of the liquid?
Asked by | 02 Sep, 2011, 02:48: PM
Surface tension, represented by the symbol ? is defined as the force along a line of unit length, where the force is parallel to the surface but perpendicular to the line.
The cohesive forces among the liquid molecules are responsible for this phenomenon of surface tension. In the bulk of the liquid, each molecule is pulled equally in every direction by neighboring liquid molecules, resulting in a net force of zero. The molecules at the surface do not have other molecules on all sides of them and therefore are pulled inwards. This creates some internal pressure and forces liquid surfaces to contract to the minimal area.
Answered by | 05 Sep, 2011, 09:55: PM
Concept Videos
CBSE 11-science - Physics
Asked by rajuinwati12 | 04 Mar, 2024, 09:22: AM
CBSE 11-science - Physics
Asked by joelisaacsam | 09 Jan, 2024, 06:31: AM
CBSE 11-science - Physics
Asked by rupimadhes | 21 Nov, 2023, 05:31: AM
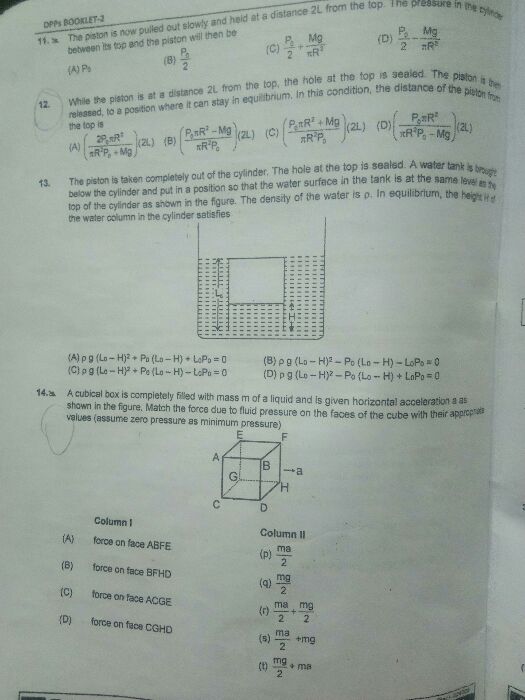
CBSE 11-science - Physics
Asked by arjunsah797 | 12 Mar, 2022, 11:10: AM
CBSE 11-science - Physics
Asked by suragimathpraveen2001 | 10 Jan, 2022, 08:06: PM
CBSE 11-science - Physics
Asked by raghuvanshisaumya461 | 03 Dec, 2021, 08:05: AM
CBSE 11-science - Physics
Asked by abhinavakrishna1009 | 31 Mar, 2021, 11:15: AM
CBSE 11-science - Physics
Asked by vanshraaj.ind | 13 Dec, 2020, 06:06: PM
CBSE 11-science - Physics
Asked by ashanihalchandani | 26 May, 2020, 09:11: AM
CBSE 11-science - Physics
Asked by mahimanaik290 | 10 May, 2020, 12:45: AM