CBSE Class 11-science Answered
Discuss the shape of CH3+ ion?
Asked by Topperlearning User | 28 Jun, 2016, 05:08: PM
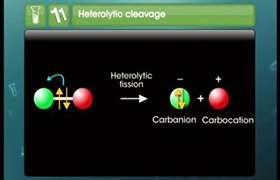
The shape of methyl carbonium ion is derived from the overlap of three equivalent C(sp2) hybridized orbitals with 1s orbital of each of the three hydrogen atoms. Each bond may be represented as C(sp2)-H(1s) sigma bond. The remaining carbon orbital is perpendicular to the molecular plane and contains no electrons.
Shape of methyl cation
Answered by | 28 Jun, 2016, 07:08: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by josephineanto1960 | 28 Mar, 2024, 12:50: PM
CBSE 11-science - Chemistry
Asked by up558486 | 28 Oct, 2021, 12:41: PM
CBSE 11-science - Chemistry
Asked by deepthibai74 | 01 Mar, 2021, 11:13: AM
CBSE 11-science - Chemistry
Asked by bhaleraoshweta237 | 20 Oct, 2020, 09:29: PM
CBSE 11-science - Chemistry
Asked by Subramanian.jayanth | 16 Jun, 2020, 10:39: AM
CBSE 11-science - Chemistry
Asked by kpbhake | 19 Oct, 2018, 06:44: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 18 Jul, 2014, 09:17: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM