CBSE Class 11-science Answered
discovery of electron
Asked by sunitasingh10412 | 20 Jul, 2020, 02:58: PM
Discovery of Electrons-
Cathode Rays
- When a high-voltage electric current is passed through the discharge tube containing a gas at a very low pressure, a green fluorescence is seen coming out of the other end of the discharge tube.
- This fluorescence is the result of rays emitted from the cathode (negative plate) towards the anode (positive plate) in the discharge tube. Hence, these rays are called cathode rays.
- Later, J. J. Thomson studied the characteristics and the constituents of cathode rays.
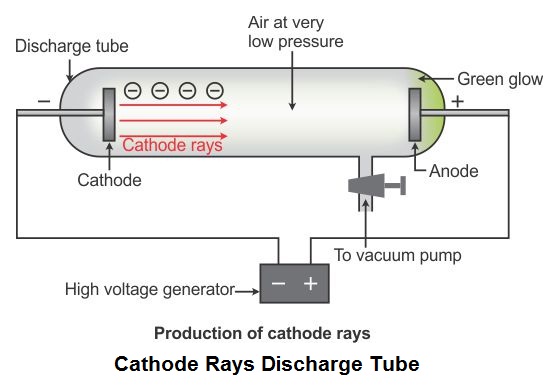
Conclusion: From his experiment, Thomson arrived at the conclusion that
1. Cathode rays are nothing but a stream of negatively charged particles called electrons.
2. These negatively charged particles are an integral part of all atoms.
3. Electrons have both definite mass and definite electric charge, both of which are independent of the nature of the gas in the discharge tube.
Properties of Electrons
- Electrons from al the sources are alike, having identical mass.
- They are constituent part of all atoms.
- An electron carries negative charge of magnitude -1.602×10-19 Coulombs.
- Its mass is 1/1837 the mass of hydrogen atom, which is 9.108×10-31 kg.
- An electron is extremely small, its radius is less than 1×10-15 m
Answered by Ravi | 20 Jul, 2020, 07:06: PM
Application Videos
Concept Videos
CBSE 11-science - Chemistry
Asked by ammu32811 | 20 Feb, 2024, 08:58: AM
CBSE 11-science - Chemistry
Asked by kv3582976 | 11 Oct, 2023, 06:57: AM
CBSE 11-science - Chemistry
Asked by dhondesainath | 28 Sep, 2021, 07:45: AM
CBSE 11-science - Chemistry
Asked by jaidawra48 | 13 Aug, 2021, 06:30: PM
CBSE 11-science - Chemistry
Asked by aayushiyadav408 | 12 Jul, 2021, 03:26: PM
CBSE 11-science - Chemistry
Asked by anandkumar10.12.96 | 28 Dec, 2020, 02:34: PM
CBSE 11-science - Chemistry
Asked by fizajain21 | 18 Dec, 2020, 12:46: PM
CBSE 11-science - Chemistry
Asked by darshanabhamare72 | 16 Oct, 2020, 06:31: PM
CBSE 11-science - Chemistry
Asked by brahampreetkaur818 | 15 Oct, 2020, 10:13: PM
CBSE 11-science - Chemistry
Asked by anish.4006 | 18 Sep, 2020, 08:00: PM





