CBSE Class 11-science Answered
difference between esters and peroxides?
Asked by | 27 Feb, 2013, 01:25: AM
A peroxide is a compound containing an oxygenoxygen single bond or the peroxide anion ([O?O]2). The O?O group is called the peroxide group or peroxo group. In contrast to oxide ions, the oxygen atoms in the peroxide ion have an oxidation state of ?1.
Esters on the other hand have general molecular formula 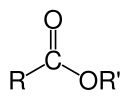 .
.
 .
.
Answered by | 27 Feb, 2013, 09:58: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by josephineanto1960 | 28 Mar, 2024, 12:50: PM
CBSE 11-science - Chemistry
Asked by neet2025targetgo | 25 Mar, 2024, 10:13: AM
CBSE 11-science - Chemistry
Asked by ap4450962 | 12 Mar, 2024, 07:35: PM
CBSE 11-science - Chemistry
Asked by pamjat.8888 | 31 Jan, 2024, 11:31: AM
CBSE 11-science - Chemistry
Asked by sahumahesh3973 | 20 Jan, 2024, 06:33: PM
CBSE 11-science - Chemistry
Asked by aswintj2007 | 07 Jan, 2024, 08:53: PM
CBSE 11-science - Chemistry
Asked by dipalisingh0908 | 05 Nov, 2023, 02:24: PM
CBSE 11-science - Chemistry
Asked by badalbehera258369 | 19 Oct, 2023, 02:01: PM
CBSE 11-science - Chemistry
Asked by prakrutikhosla | 16 Sep, 2023, 06:31: PM
CBSE 11-science - Chemistry
Asked by shahintkjnv2016 | 13 Jun, 2022, 07:17: PM











