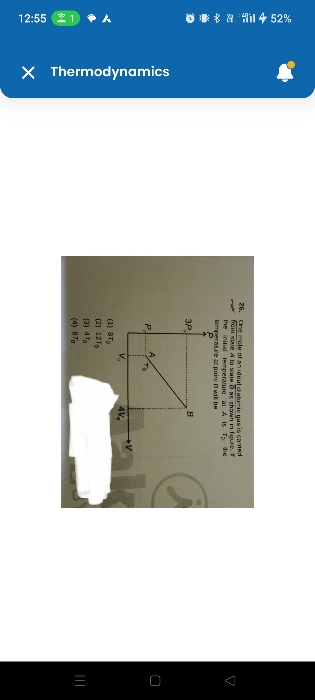
CBSE Class 11-science Answered
No external work is being done when a gas is heated at constant volume i.e. gas uses all the haet which is given to it for increasing itsinternal energy. Hence if temperature of one mole of a gas is raised through 1oC, the molar heat capacity is given itself at constant volume by increase in internal energy.
But when a gas is heated at constant pressure there will be expansion of gas i.e. increase in volume take place and some external work will b done. For this some extra heat is required which should be given to the gas to perform the external work.
Hence the Molar Heat capacity of a gas at constant pressure must be greater than Molar Heat capacity of a gas at constant volume.
Sometimes a process takes place relatively slowly. Slowly enough so that all the intensive variables can have actually definite values through the entire path taken by the process. Such a process is called a quasi-static process.
For example, let us assume that our system is an amount of a gas trapped inside a cylinder, pushing on a piston forming one end of the cylinder. If the piston moves out rapidly, the gas expands rapidly and the pressure will vary from point to point inside the gas. But if the piston moves very very slowly, possibly due to friction or some artificial restriction limiting its speed, the pressure may well have a common value throughout the system at every instant in time.
The time scale involved is easy to figure. If the piston moves rapidly, pressure waves will exist inside the cylinder and there will be no one pressure for the system. But if the piston moves slowly, compared to the speed of sound in the gas in the cylinder for example, then the pressure will be essentially uniform all the time. Such a process is a quasi-static one.
The advantage of a quasi-static process is that at each point along the path taken by the system in going from the initial state to its final state the system is effectively at equilibrium. So we can define this path very precisely.