CBSE Class 10 Answered
Describe an activity to show the formation of an ester in the school laboratory.
Asked by Topperlearning User | 05 Feb, 2015, 02:01: PM
Aim - To show the formation of ester.
Requirements- test tube, beaker, burner, alcohol, acetic acid, conc. H2SO4
Theory:

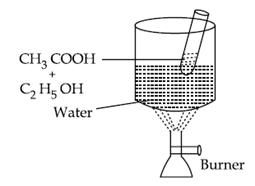
Procedure
(a) A test tube was taken in which ethanol and ethanoic acid was taken.
(b) To this, Conc. H2SO4 is added.
(c)The test tube is kept in a beaker containing water.
Observation: A fruity smell comes out.
Result: Ester is formed
Precautions:
(a) Direct heating is avoided.
(b) Only a few drop of conc. H2SO4 should be added.
Answered by | 05 Feb, 2015, 04:01: PM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by sneh | 27 Mar, 2020, 10:11: AM
CBSE 10 - Chemistry
Asked by pranjaliinamdar2004 | 29 Feb, 2020, 07:20: PM
CBSE 10 - Chemistry
Asked by sweetykhatri99254 | 27 Feb, 2020, 03:40: PM
CBSE 10 - Chemistry
Asked by priyanshiishu | 30 Jan, 2020, 10:39: AM
CBSE 10 - Chemistry
Asked by kamalnayansingh7 | 13 Jan, 2020, 08:35: AM
CBSE 10 - Chemistry
Asked by Deepak | 22 Dec, 2019, 11:20: PM
CBSE 10 - Chemistry
Asked by ritikraghuwanshi6986 | 16 Dec, 2019, 08:42: PM
CBSE 10 - Chemistry
Asked by vedantsagrawal23 | 05 Dec, 2019, 08:34: AM
CBSE 10 - Chemistry
Asked by aryasaxena2003 | 25 Jul, 2019, 05:35: PM
CBSE 10 - Chemistry
Asked by rushabhjain.avv | 21 Mar, 2019, 10:07: PM