CBSE Class 11-science Answered
Define hybridization. Show the formation of ammonia (NH3) molecule.
Asked by Topperlearning User | 13 Jun, 2016, 02:25: PM
i) It can be defined as the process of intermixing of the orbitals of slightly different energies so as to redistribute their energies, resulting in the formation of new set of orbitals of equivalent energies and shape.
ii) Formation of ammonia molecule:
In ammonia molecule, the nitrogen atom is sp3 hybridized.
Three sp3-hybrid orbitals of N atom are used for forming sp3-s 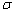 (sigma) bonds with H atoms.
(sigma) bonds with H atoms.
The fourth sp3-hybrid orbital carry lone pair of electrons.
The relatively larger lp-bp interactions cause H-N-H angle to decrease from 109o 28' to 107o.
Answered by | 13 Jun, 2016, 04:25: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by Trisha Gupta | 30 Oct, 2022, 05:36: PM
CBSE 11-science - Chemistry
Asked by ABHILASHA | 22 Aug, 2020, 04:39: AM
CBSE 11-science - Chemistry
Asked by kpbhake | 12 Mar, 2018, 11:45: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 08 Oct, 2014, 01:09: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 13 Jun, 2016, 02:26: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 11-science - Chemistry
What is the hybrid state of B in BF3, Al in AlCl3, Be in BeCl2, C in CO2 and C2H4; S in SO2 and SO3.
Asked by Topperlearning User | 08 Oct, 2014, 01:33: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 09 Oct, 2014, 09:30: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM


 on the basis of hybridisation
on the basis of hybridisation