CBSE Class 11-science Answered
Define and explain the law of chemical equilibrium.
Asked by Topperlearning User | 16 Jun, 2016, 05:17: PM
The law of chemicalequilibrium states that in a reversible reaction the ratio of the rate of the forward reaction to the rate of the reverse reaction is a constant for that reaction at a constant temperatuere.
For a general chemical reaction
a A + b B  c C + d D
c C + d D
 c C + d D
c C + d DAccording to the law of mass action : rf =kf [A]a [B]b and rb = kb[C]c [D]d
At equilibrium point , rf = rb
Or,kf [A]a [B]b = kb[C]c [D]d Or, kf/ kb = KC
|
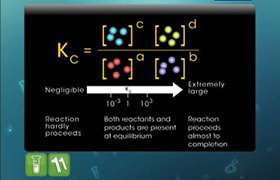
KC =
|
[C]c [D]d
[A]a [B]b |
Where Kc is the equilibrium constant and the notation [A] signifies the molar concentration of species A. An alternative expression for the equilibrium constant for reaction involving gaseous substances ( concentration expressed in partial pressure) is as below
|
KP =
|
PCc PDd
PAa PBb |
Answered by | 16 Jun, 2016, 07:17: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by visank90 | 24 Nov, 2023, 10:45: AM
CBSE 11-science - Chemistry
Asked by rakhikumarithakur4 | 22 May, 2020, 08:21: PM
CBSE 11-science - Chemistry
Asked by arshrana3272 | 04 Mar, 2020, 02:57: PM
CBSE 11-science - Chemistry
Asked by hsdhall.2005 | 12 Nov, 2019, 11:40: PM
CBSE 11-science - Chemistry
Asked by govtsecschoolnayaganv051 | 19 Jul, 2018, 08:52: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 16 Jun, 2016, 05:17: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 16 Jun, 2016, 05:17: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 27 Apr, 2015, 03:08: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 27 Apr, 2015, 04:12: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 27 Apr, 2015, 04:15: PM




 6H2O (g) + 2N2 (g) How will the equilibrium shift if: a) The volume is increased, b) Helium gas is added
6H2O (g) + 2N2 (g) How will the equilibrium shift if: a) The volume is increased, b) Helium gas is added