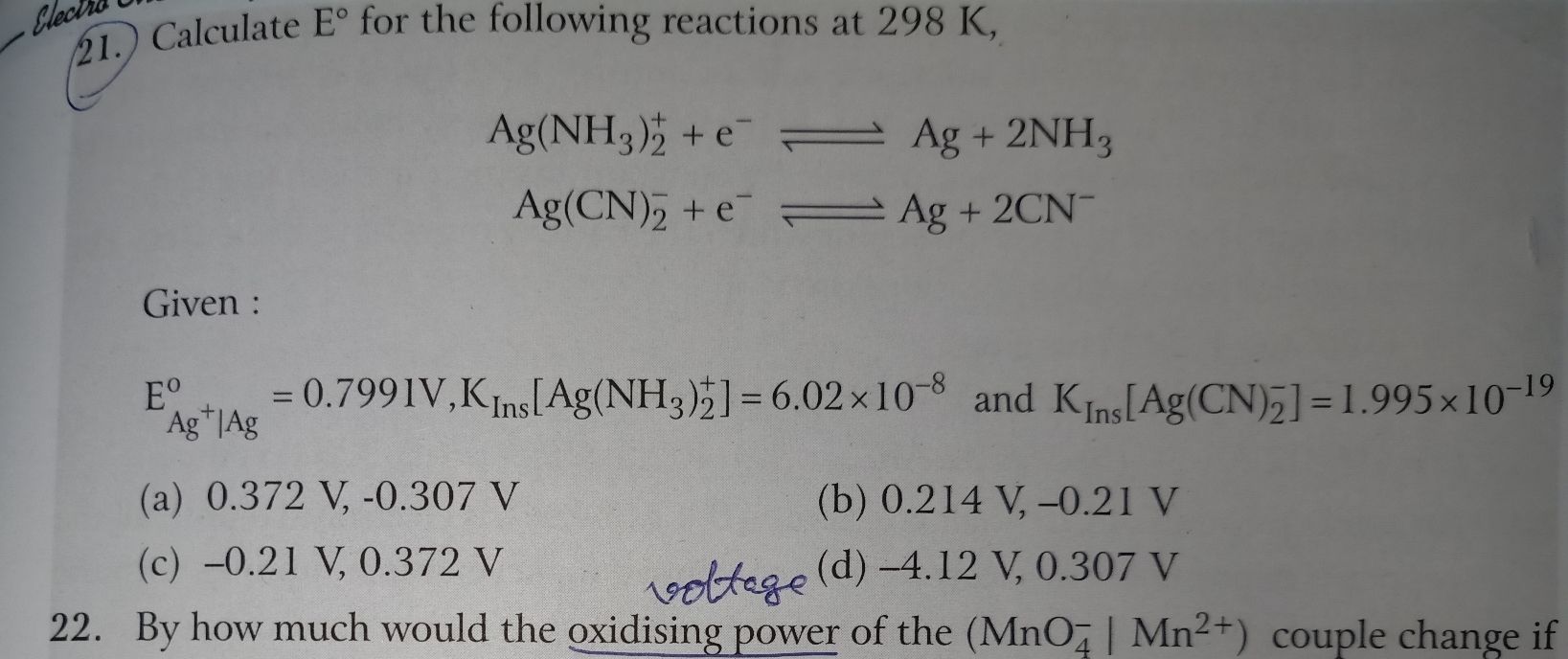CBSE Class 12-science Answered
cu2+ + 2e- = cu and h+ + 2e= 1/2h2 which is more feasible and why ?
Asked by Gishin Rajan | 17 Dec, 2015, 10:40: PM
The standard reduction potential for the reduction of copper is 0.34 V and that for the reduction of hydrogen is 0.0 V.
Higher reduction potential means that reduction reaction is more feasible.
Hence, Cu2+ + 2e- → Cu is more feasible.
Answered by Prachi Sawant | 20 Dec, 2015, 02:53: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by yashwanthgowdakn4 | 22 Feb, 2024, 09:14: PM
CBSE 12-science - Chemistry
Asked by keerthana.d.cst.2022 | 22 Aug, 2023, 08:18: PM
CBSE 12-science - Chemistry
Asked by amankumar2004112 | 04 May, 2022, 01:12: PM
CBSE 12-science - Chemistry
Asked by Balbir | 02 Oct, 2019, 12:29: AM
CBSE 12-science - Chemistry
Asked by Jsiajaijiaia | 27 Aug, 2019, 12:23: AM
CBSE 12-science - Chemistry
Asked by prakriti12oct | 14 Aug, 2019, 12:46: AM
CBSE 12-science - Chemistry
Asked by rakeshraghav33 | 19 May, 2018, 03:55: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 22 Jun, 2016, 02:42: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 22 Jun, 2016, 02:41: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 22 Jun, 2016, 02:41: PM