CBSE Class 12-science Answered
Coordination compounds
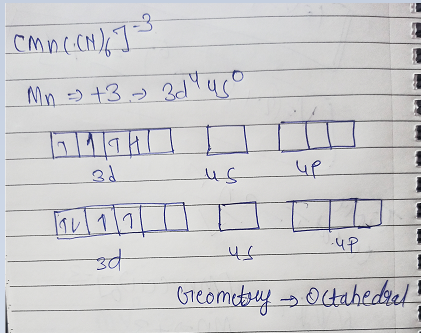
predict the hybridisation and geometry of [Mn F6]3- on the basis of valence bond theory
with diagram showing electron filling.
Asked by fishtailfever | 21 Feb, 2021, 02:07: PM
Hybridistation of [MnF6]3- is d2sp3. Gemoetry-Octahedral
Answered by Ravi | 21 Feb, 2021, 10:44: PM
CBSE 12-science - Chemistry
Asked by navadeepnavadeep242 | 19 Mar, 2024, 08:56: PM
CBSE 12-science - Chemistry
Asked by arjunsah797 | 10 May, 2022, 12:16: PM
CBSE 12-science - Chemistry
Asked by shivubh161 | 24 May, 2021, 03:39: PM
CBSE 12-science - Chemistry
Asked by fishtailfever | 21 Feb, 2021, 02:07: PM
CBSE 12-science - Chemistry
Asked by jain.pradeep | 27 Feb, 2020, 02:50: PM
CBSE 12-science - Chemistry
Asked by jain.pradeep | 26 Feb, 2020, 09:59: AM
CBSE 12-science - Chemistry
Asked by kasireddyteja15 | 04 Dec, 2019, 09:52: PM
CBSE 12-science - Chemistry
Asked by jaideepsnatu | 21 Feb, 2019, 04:04: PM
CBSE 12-science - Chemistry
Asked by amitarastogijuly12 | 24 Jan, 2019, 04:48: PM
CBSE 12-science - Chemistry
Asked by govtsecschoolnayaganv051 | 30 Jul, 2018, 05:33: PM



