CBSE Class 12-science Answered
Conversion of amines
Asked by Sudamkalgunde624 | 31 Dec, 2019, 11:38: AM
Conversion of Amines:
1. Acylation reaction
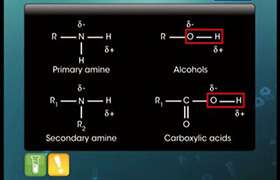
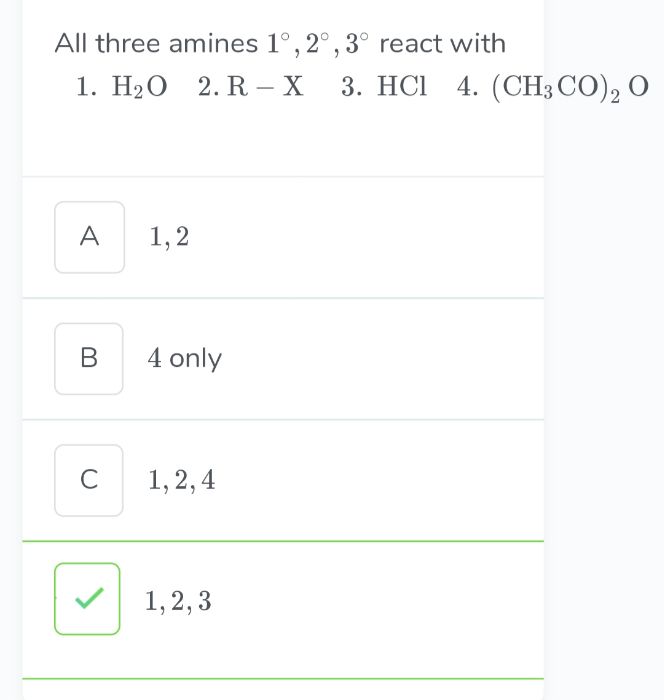
- Aliphatic and aromatic primary and secondary amines (which contain replaceable hydrogen atoms) react with acid chlorides, anhydrides and esters to form substituted amide.
- The process of introducing an acyl group (R–CO–) into the molecule is called acylation.
- The reaction is carried out in the presence of a stronger base than the amine, such as pyridine, which removes HCl formed and shifts the equilibrium to the product side.

2. Carbylamine reaction
- On heating aliphatic and aromatic primary amines with chloroform and ethanolic KOH they form isocyanides or carbylamines which have foul odour.
- Secondary and tertiary amines do not show this reaction.
- This reaction is used as a test for primary amines.
Answered by Varsha | 31 Dec, 2019, 03:30: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by kaziryan.05 | 06 Jul, 2021, 11:31: PM
CBSE 12-science - Chemistry
Asked by mufeedatvp2000 | 15 Apr, 2020, 01:35: PM
CBSE 12-science - Chemistry
Asked by Sudamkalgunde624 | 31 Dec, 2019, 11:38: AM
CBSE 12-science - Chemistry
Asked by prakriti12oct | 19 Nov, 2019, 12:39: PM
CBSE 12-science - Chemistry
Asked by dineshchem108 | 19 Jun, 2019, 09:19: PM
CBSE 12-science - Chemistry
Asked by afiaorpi01 | 22 Mar, 2019, 01:19: AM
CBSE 12-science - Chemistry
Asked by abhitailor158 | 07 Mar, 2019, 04:44: PM
CBSE 12-science - Chemistry
Asked by Atulcaald | 16 May, 2018, 02:44: PM




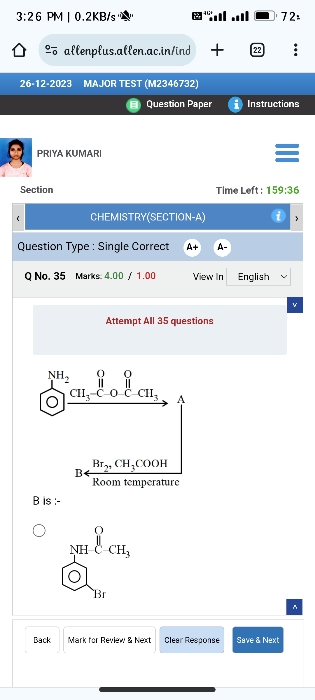
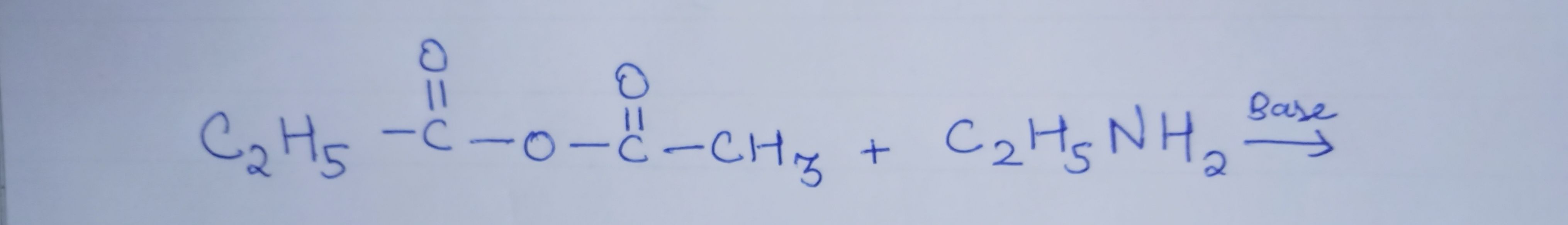
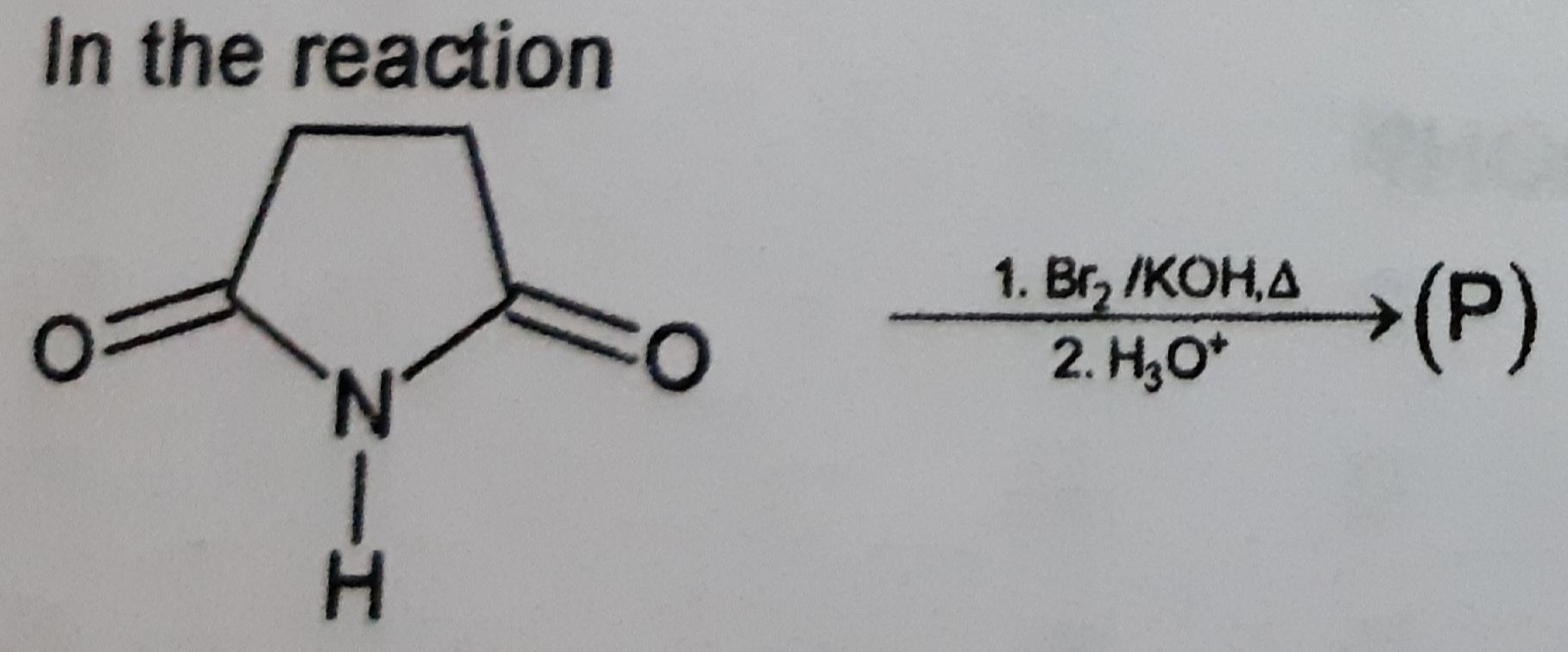

 Z is :-
(1)
CH3—CH2—OH
(2)
CH3—NH2
(3)
CH3—OH
(4)
CH3—CH2—NH2
Z is :-
(1)
CH3—CH2—OH
(2)
CH3—NH2
(3)
CH3—OH
(4)
CH3—CH2—NH2