CBSE Class 12-science Answered
consider a hypothesis H-like atom, the wavelength in Amstrong for the spectral lines for the transition from n=p to n=1 is given as
wavelength =1500p^2/p^2 -1 where p>1
find the minimum and maximum wavelength
Asked by Akshatkuma2004 | 04 Jul, 2021, 04:04: PM
Wavelength λ is given as
λ = 1500 × 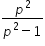 , where p > 1
, where p > 1
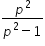 , where p > 1
, where p > 1Units for the constant 1500 is not known .
we get maximum wavelength when p =2 , hence 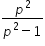 = (4/3) and λ = 2000
= (4/3) and λ = 2000
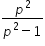 = (4/3) and λ = 2000
= (4/3) and λ = 2000Minimum wavelegth is obtained when p >> 1 , so that 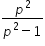 ≈ 1 and λ = 1500
≈ 1 and λ = 1500
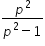 ≈ 1 and λ = 1500
≈ 1 and λ = 1500
Answered by Thiyagarajan K | 04 Jul, 2021, 05:30: PM
CBSE 12-science - Physics
Asked by dasrituparna1999 | 12 Apr, 2024, 09:26: PM
CBSE 12-science - Physics
Asked by Akshatkuma2004 | 04 Jul, 2021, 04:04: PM
CBSE 12-science - Physics
Asked by rsrakesh932 | 12 Jun, 2020, 04:38: PM
CBSE 12-science - Physics
Asked by yadavnitish688 | 14 Dec, 2019, 06:04: AM
CBSE 12-science - Physics
Asked by sidiz.shrestha07 | 26 May, 2019, 06:42: AM
CBSE 12-science - Physics
Asked by pardeepkumar2281 | 28 Feb, 2019, 07:24: PM
CBSE 12-science - Physics
Asked by pradeepjsme | 30 Jan, 2019, 03:54: PM
CBSE 12-science - Physics
Asked by pradeepjsme | 30 Jan, 2019, 03:51: PM
CBSE 12-science - Physics
Asked by rohitraman1115 | 14 Jan, 2019, 12:02: PM
CBSE 12-science - Physics
Asked by rohitraman1115 | 14 Jan, 2019, 12:01: PM

