ICSE Class 9 Answered
Compare the mass of a proton with that of an electron.
Asked by Abl123736 | 01 Jan, 2016, 09:34: PM
Mass of proton = 1,673 × 10-24 g
Mass of electron = 9.109 X 10-28 g
Hence,
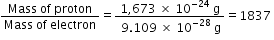
Thus, mass of the a proton is 1837 times that of an electron.
Answered by Yashvanti Jain | 02 Jan, 2016, 09:31: AM

