CBSE Class 9 Answered
Carbon
Asked by Rishav | 04 Jun, 2008, 09:13: PM
It is most conveniently prepared in the lab. by the action of an acid on a carbonate. The apparatus used is shown below. The chemicals used are usually limestone or marble chips and dilute hydrochloric acid.
CALCIUM + HYDROCHLORIC  CALCIUM + WATER + CARBON
CALCIUM + WATER + CARBON
CARBONATE ACID CHLORIDE DIOXIDE
(MARBLE)
 CALCIUM + WATER + CARBON
CALCIUM + WATER + CARBONCARBONATE ACID CHLORIDE DIOXIDE
(MARBLE)
CaCO3 + 2HCl 
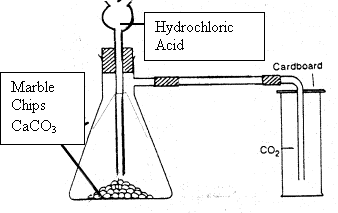
Diagram for lab preparation of carbon dioxide
Answered by | 18 Jun, 2008, 04:44: AM
Application Videos
Concept Videos
CBSE 9 - Chemistry
Asked by namratarnav123 | 21 Apr, 2024, 11:25: PM
CBSE 9 - Chemistry
Asked by mailtoparvathyprajith | 12 Feb, 2024, 10:08: AM
CBSE 9 - Chemistry
Asked by rajputanaji290 | 03 Oct, 2023, 09:30: PM
CBSE 9 - Chemistry
Asked by Premkharra7568 | 18 Aug, 2023, 04:41: PM
CBSE 9 - Chemistry
Asked by rubinapathan228 | 29 Jun, 2023, 05:45: PM
CBSE 9 - Chemistry
Asked by yforyt3672 | 09 Apr, 2023, 07:37: PM
CBSE 9 - Chemistry
Asked by muditsharma287 | 09 Mar, 2023, 10:10: PM
CBSE 9 - Chemistry
Asked by shivalaxmi0205 | 08 Mar, 2023, 07:47: PM
CBSE 9 - Chemistry
Asked by shivalaxmi0205 | 08 Mar, 2023, 07:46: PM











