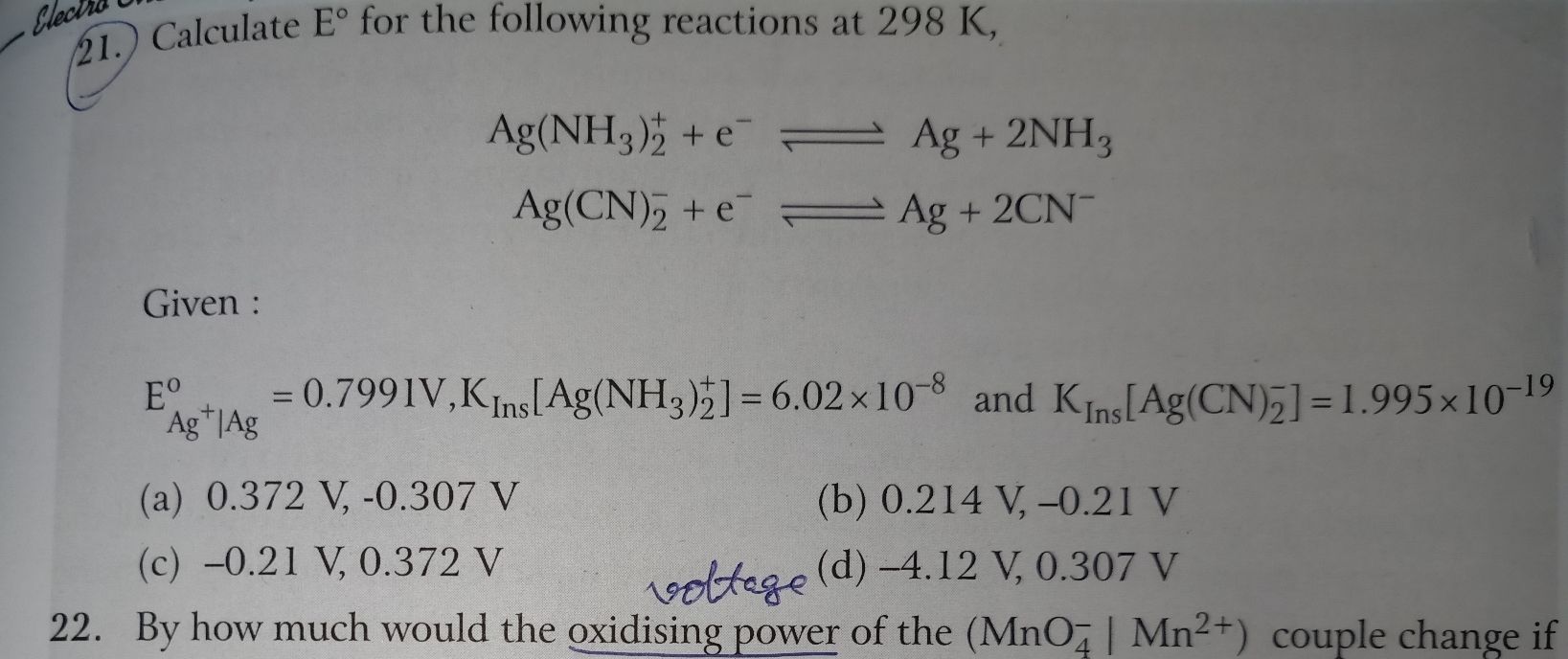CBSE Class 12-science Answered
The potential of a single electrode in a half-cell is called the Single electrode potential.
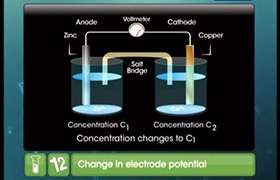
In Daniel cell in which the electrodes are not connected externally, the anode Zn/Zn2+ develops a negative charge and the cathode Cu/Cu2+, a positive charge.
The amount of the charge produced on an individual electrode determines its single electrode potential.
Nernst's equation for single electrode is

where
[M] = Concentration of the metal say M
[M n + ] = Concentration of oxidized state of the metal.
E = Electrode potential at concentration M n +.
E = Standard electrode potential.
R= Gas constant
T= Temperature in K
F= 1 Faraday (96500 coulombs)
n = Number of electrons involved in the electrode reaction.