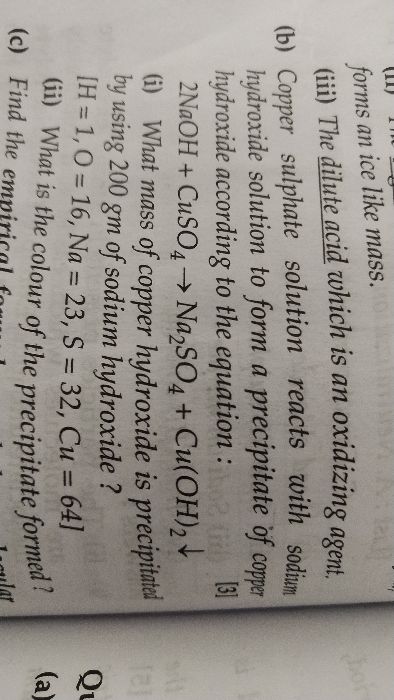ICSE Class 10 Answered
can you please explain numericals based on gaylussac's law briefly?
Asked by savi1405 | 28 Apr, 2016, 05:02: PM
Gay-Lussac's Law of combining volumes: When gases react, they do so in volumes which bear a simple ratio to one anothet, and to the volume of the gaseous product, provided that all the volumes are measured at the same temperature and pressure.
Numerical example:
Problem: 40 cm3 of methane is mixed with 100 cm3 of pure oxygen at the same temperature and pressure. The mixture is then ignited. Calculate the composition of resulting mixtureif it is cooled to initial room temperature and pressure.
CH4 + 2O2 → CO2 + 2H2O
Solution:

Answered by Prachi Sawant | 29 Apr, 2016, 10:35: AM
Application Videos
Concept Videos
ICSE 10 - Chemistry
Asked by jrvedant208 | 05 Feb, 2024, 10:37: PM
ICSE 10 - Chemistry
Asked by rashikulkarni28 | 10 Jul, 2022, 10:13: PM
ICSE 10 - Chemistry
Asked by rashikulkarni28 | 25 Jun, 2022, 10:24: PM
ICSE 10 - Chemistry
Asked by palshivom72 | 14 Jul, 2020, 07:56: PM
ICSE 10 - Chemistry
Asked by jhabijay01 | 27 May, 2020, 12:20: PM
ICSE 10 - Chemistry
Asked by aashimegh | 04 Sep, 2019, 08:53: AM
ICSE 10 - Chemistry
Asked by aashimegh | 04 Sep, 2019, 08:37: AM
ICSE 10 - Chemistry
Asked by aashimegh | 28 Aug, 2019, 05:25: PM