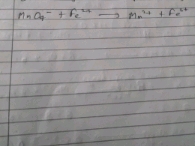CBSE Class 11-science Answered
can you explain the concept of liquefaction of gases, and the various methods of acheiving it ?
Asked by | 12 Apr, 2008, 09:05: PM
Gases have a lot of intermolecular distances. By reducing the temperature the K.E reduses, if the temperature reduced below the critical temperature of a particular gas it changes to liquid. If the Pressure alone is increased the gas compresses the vanderwaal forces act and gas liquifies above the critical pressure.
Compression brings the molecules in close vicinity and cooling slows down the moment of molecules therefore , intermolecular interactions hold the closely and slowly moving molecules together and the gas liquifies.
Answered by | 07 May, 2008, 07:00: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by hm6561889 | 15 Apr, 2024, 07:45: AM
CBSE 11-science - Chemistry
Asked by manikandanragul1 | 11 Apr, 2024, 09:02: AM
CBSE 11-science - Chemistry
Asked by jayag1298 | 08 Apr, 2024, 03:14: PM
CBSE 11-science - Chemistry
Asked by omniscientnjf2021 | 07 Apr, 2024, 10:18: PM
CBSE 11-science - Chemistry
Asked by ansh.skulkarni1158 | 07 Apr, 2024, 11:03: AM
CBSE 11-science - Chemistry
Asked by nikhithaguguloth14 | 29 Mar, 2024, 08:15: PM
CBSE 11-science - Chemistry
Asked by sumedhasingh238 | 27 Mar, 2024, 11:04: PM
CBSE 11-science - Chemistry
Asked by avijotsingh946431 | 22 Feb, 2024, 05:36: PM
CBSE 11-science - Chemistry
Asked by gurmelsinghray | 21 Feb, 2024, 08:43: AM
CBSE 11-science - Chemistry
Asked by VarunTYAGi9013 | 21 Oct, 2023, 07:14: PM