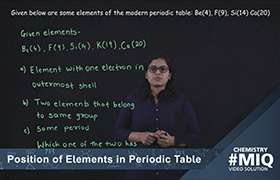
CBSE Class 10 Answered
can u give me a table of the trends in modern periodic table that is what increases and decreses across the period and down the group...and can u plz tell me how to remember them in a better or a faster way !! thank you
Asked by Chandana kiran | 27 Feb, 2015, 08:44: PM
Trends or Periodicity
The periodicity of these properties follows trends as you move across a row or period of the periodic table or down a column or group:
Moving Left → Right
- Ionization Energy Increases
- Electronegativity Increases
- Atomic Radius Decreases
Moving Top → Bottom
- Ionization Energy Decreases
- Electronegativity Decreases
- Atomic Radius Increases
Tips to study the modern periodic table
- Print out a copy of the periodic table and stick it near your study desk.
- Breakdown the table into smaller sections to learn it. Most charts are already divided by color and type of element. You could go by row, column, atomic weight, or simply easiest to hardest. Find patterns that stick out to you.
- Start with the s-block elements first. Then try to learn the p-block elements groupwise. Later, start learning lanthanides and actinides.
- Keep a copy of a periodic table in your wallet. Try learning the periodic table when you get free time. For example, traveling by public transport or just waiting in the line for something.
- Learn a few every day and always review the old ones! If you don't review and quiz yourself, you will forget.
- You can also learn the periodic table by catchy song or a sentence. You can either create your own or go on the internet.
- Learn all the trends of the modern periodic table.
- Refer to the chapter notes provided by www.topperlearning.com
Answered by Hanisha Vyas | 27 Feb, 2015, 08:59: PM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by dhumalchhaya13 | 26 May, 2022, 11:05: PM
CBSE 10 - Chemistry
Asked by kanchanbalpande82 | 12 Apr, 2022, 11:01: AM
CBSE 10 - Chemistry
Asked by kanchanbalpande82 | 12 Apr, 2022, 11:01: AM
CBSE 10 - Chemistry
Asked by waghmaresheetal78 | 27 Dec, 2021, 05:02: PM
CBSE 10 - Chemistry
Asked by ksheera36 | 03 Jun, 2021, 08:35: PM
CBSE 10 - Chemistry
Asked by vungtsaniyanthan | 16 May, 2021, 06:32: PM
CBSE 10 - Chemistry
Asked by sinhagopalakumara | 01 May, 2021, 08:16: PM
CBSE 10 - Chemistry
Asked by advssdrall | 26 Mar, 2021, 07:43: AM
CBSE 10 - Chemistry
Asked by shettyshrinidhi271 | 07 Jan, 2021, 05:04: PM
CBSE 10 - Chemistry
Asked by adipadmakarri | 05 Dec, 2020, 06:41: PM