CBSE Class 11-science Answered
Calculate the pressure of 2 mol of ammonia at 27 C when its volume is 5 litres according to van der Waals equation? (Given that a = 4.17, b = 0.03711 and R=0.082)
Asked by mushirateli33.11dga | 01 Dec, 2021, 07:13: PM
Given:
n = 2, V = 5 litres
T = 273 + 27 = 300 K
R = 0.082 lit atm deg-1 mole-1
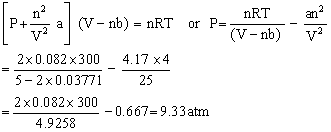
Answered by Ramandeep | 01 Dec, 2021, 09:17: PM
Application Videos
Concept Videos
CBSE 11-science - Chemistry
Asked by rhythmdraco42 | 22 Apr, 2024, 10:43: PM
CBSE 11-science - Chemistry
Asked by hm6561889 | 15 Apr, 2024, 07:45: AM
CBSE 11-science - Chemistry
Asked by manikandanragul1 | 11 Apr, 2024, 09:02: AM
CBSE 11-science - Chemistry
Asked by jayag1298 | 08 Apr, 2024, 03:14: PM
CBSE 11-science - Chemistry
Asked by omniscientnjf2021 | 07 Apr, 2024, 10:18: PM
CBSE 11-science - Chemistry
Asked by ansh.skulkarni1158 | 07 Apr, 2024, 11:03: AM
CBSE 11-science - Chemistry
Asked by nikhithaguguloth14 | 29 Mar, 2024, 08:15: PM
CBSE 11-science - Chemistry
Asked by josephineanto1960 | 28 Mar, 2024, 12:50: PM
CBSE 11-science - Chemistry
Asked by sumedhasingh238 | 27 Mar, 2024, 11:04: PM
CBSE 11-science - Chemistry
Asked by neet2025targetgo | 25 Mar, 2024, 10:13: AM













