CBSE Class 11-science Answered
Calculate the number of sigma & pi bonds in the following:-
2-Methylprop-1-yn
Asked by pksunilnair | 12 Mar, 2017, 08:30: PM
Dear pksunilnair@rediffmail.com
Thanks for asking us a question in Ask the Expert section of TopperLearning.com.
The provided name seems incorrect.
The correct name should be 2-methylprop-1-ene.
In this compound, there are total 11 sigma bonds (three C-C, eight C-H) and 1 pi bond (C-C).
Regards
Topperlearning Team.
Regards
Topperlearning Team.
Answered by Prachi Sawant | 13 Mar, 2017, 05:45: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by neet2025targetgo | 25 Mar, 2024, 10:13: AM
CBSE 11-science - Chemistry
Asked by pamjat.8888 | 31 Jan, 2024, 11:31: AM
CBSE 11-science - Chemistry
Asked by sahumahesh3973 | 20 Jan, 2024, 06:33: PM
CBSE 11-science - Chemistry
Asked by aswintj2007 | 07 Jan, 2024, 08:53: PM
CBSE 11-science - Chemistry
Asked by dipalisingh0908 | 05 Nov, 2023, 02:24: PM
CBSE 11-science - Chemistry
Asked by badalbehera258369 | 19 Oct, 2023, 02:01: PM
CBSE 11-science - Chemistry
Asked by prakrutikhosla | 16 Sep, 2023, 06:31: PM
CBSE 11-science - Chemistry
Asked by hitanshu04 | 17 Jun, 2021, 07:20: PM
CBSE 11-science - Chemistry
Asked by ABHILASHA | 22 Aug, 2020, 05:13: AM
CBSE 11-science - Chemistry
Asked by ABHILASHA | 22 Aug, 2020, 05:09: AM








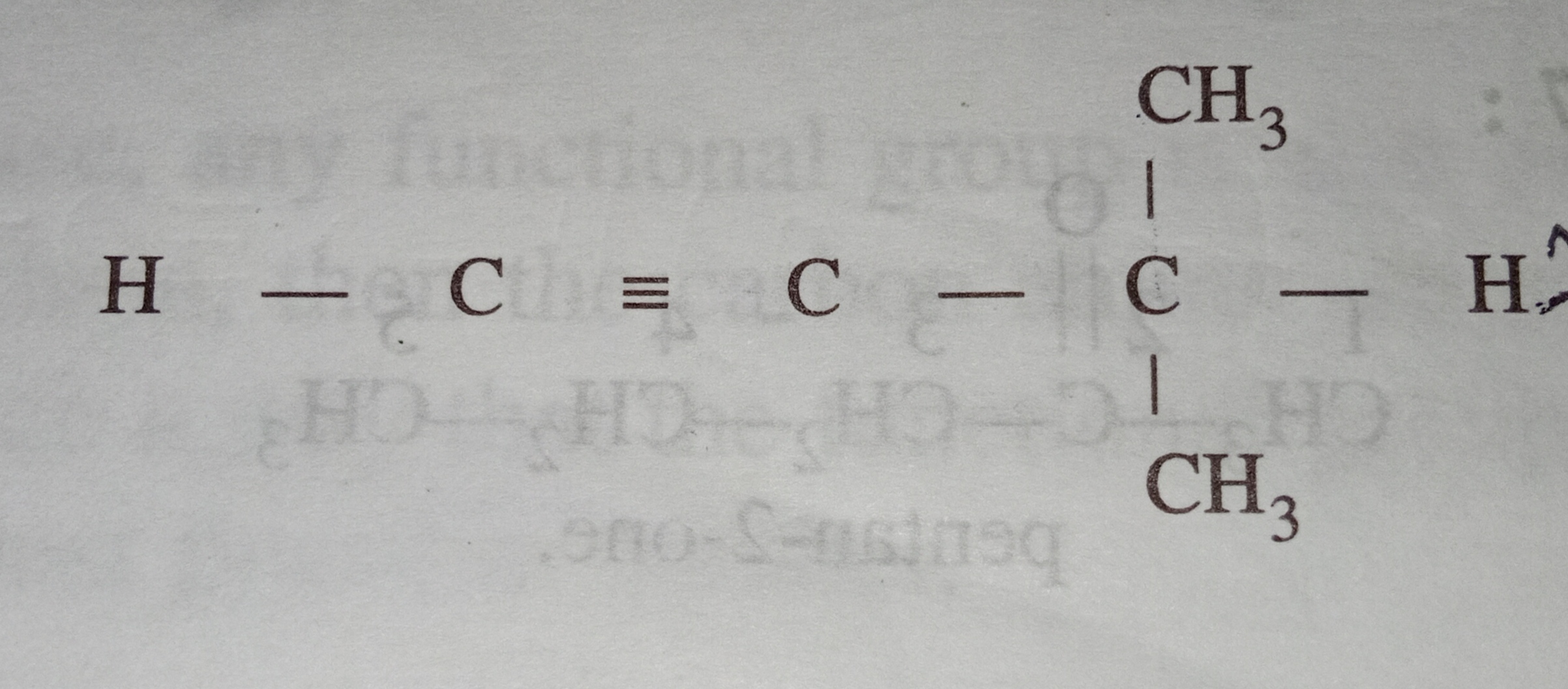
 hybridised ,how will it be classified?
hybridised ,how will it be classified?