CBSE Class 11-science Answered
Calculate the number of moles of  which contain 8.00 grams of
which contain 8.00 grams of  .
.
 which contain 8.00 grams of
which contain 8.00 grams of  .
.
Asked by aashimegh | 28 Aug, 2019, 05:28: PM
1 molecule of CO2 will have= 32 g of O2
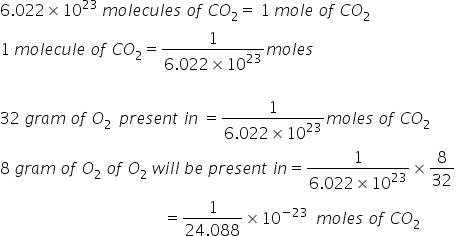
Answered by Ravi | 29 Aug, 2019, 11:17: AM
CBSE 11-science - Chemistry
Asked by ayushmishradbb | 28 Mar, 2020, 04:05: PM
CBSE 11-science - Chemistry
Asked by aashimegh | 28 Aug, 2019, 05:28: PM
CBSE 11-science - Chemistry
Asked by sanyammahajan2003 | 10 May, 2019, 07:31: PM

