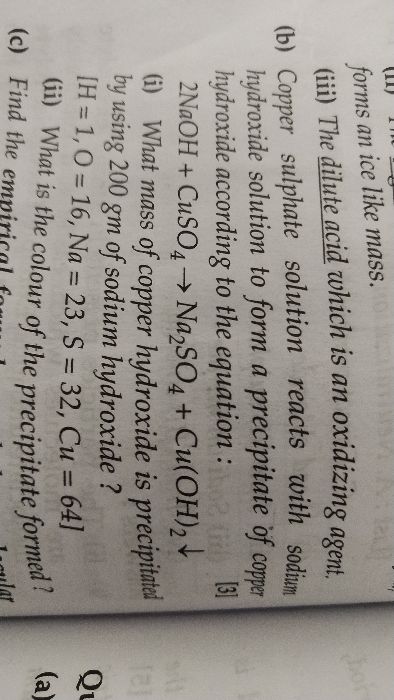ICSE Class 10 Answered
Calculate the number of ions and total charge in 3 gram CO3^2-
Asked by Anjalimanoj207 | 20 Jun, 2019, 07:43: PM
Given, Mass of carbonate = 3g
As we know,
The molar mass of carbonate ion is 60 g per mole.
This means mass of 1 mole of carbonate ions = 60 g
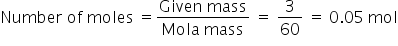
1 mole = 6.022×1023
The number of ions in 3g = 0.05×6.022×1023 = 0.3011×1023
In a carbonate ion there are 3 oxygen atoms and one carbon atom
Therefore,
Number of carbon atoms in 3g of carbonate = 1 × 0.3011×1023 =0.3011×1023
Number of oxygen atoms in 3g of carbonate = 3 × 0.3011×1023 =0.9033×1023
And the charge on carbonate ion is -2
Therefore total charge on 3g of carbonate ions = (-2) × 0.3011×1023 =-0.6022×1023
Answered by Ramandeep | 21 Jun, 2019, 12:18: PM
Application Videos
Concept Videos
ICSE 10 - Chemistry
Asked by jrvedant208 | 05 Feb, 2024, 10:37: PM
ICSE 10 - Chemistry
Asked by rashikulkarni28 | 10 Jul, 2022, 10:13: PM
ICSE 10 - Chemistry
Asked by rashikulkarni28 | 25 Jun, 2022, 10:24: PM
ICSE 10 - Chemistry
Asked by palshivom72 | 14 Jul, 2020, 07:56: PM
ICSE 10 - Chemistry
Asked by jhabijay01 | 27 May, 2020, 12:20: PM
ICSE 10 - Chemistry
Asked by aashimegh | 04 Sep, 2019, 08:53: AM
ICSE 10 - Chemistry
Asked by aashimegh | 04 Sep, 2019, 08:37: AM
ICSE 10 - Chemistry
Asked by aashimegh | 28 Aug, 2019, 05:25: PM