JEE Class main Answered
Calculate the number of grams of glycerol C3H5(OH)3(MW=92.1g/mol) , that must be dissolved in5 520 gm grams of water to raise the boiling point to 102.000c
Asked by s.ojaswini17 | 06 Feb, 2019, 01:59: AM
Given:
Molecular Weight of glycerol = 92.1 g/mol
We know,
ΔT = m× Kf
Where,
ΔT = 100 - 102
= 2 °C
m = molality of a solution
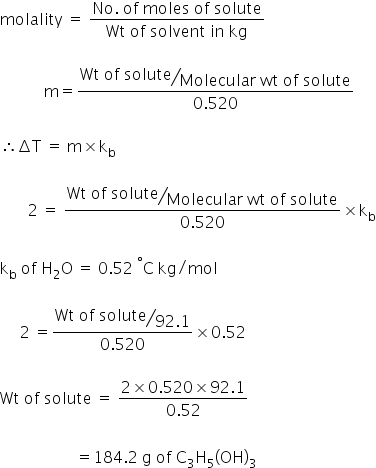
Weight of glycerol is 184.2 g.
Answered by Varsha | 06 Feb, 2019, 01:05: PM
Application Videos
Concept Videos
JEE main - Chemistry
Asked by amarnathreddyp19 | 29 Mar, 2024, 06:47: AM
JEE main - Chemistry
Asked by atharvamane801 | 14 Jan, 2024, 12:07: PM
JEE main - Chemistry
Asked by bhyogita884 | 12 Jul, 2022, 02:55: AM
JEE main - Chemistry
Asked by abdulraqeeb437 | 16 Jun, 2022, 08:38: PM
JEE main - Chemistry
Asked by akshatmi2005 | 21 May, 2021, 02:23: PM
JEE main - Chemistry
Asked by mrudulmahadev1311 | 20 Aug, 2019, 08:40: PM
JEE main - Chemistry
Asked by vishakhachandan026 | 16 Aug, 2019, 07:47: PM
JEE main - Chemistry
Asked by sunilpatil4411 | 06 Mar, 2019, 07:34: PM
JEE main - Chemistry
Asked by s.ojaswini17 | 06 Feb, 2019, 01:59: AM
JEE main - Chemistry
Asked by s.ojaswini17 | 05 Feb, 2019, 02:29: AM








