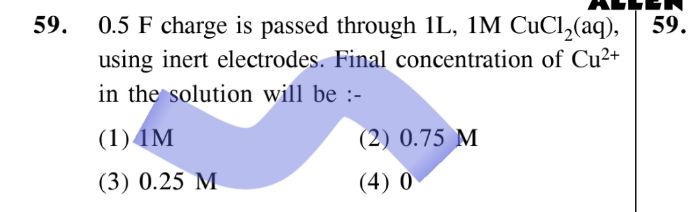
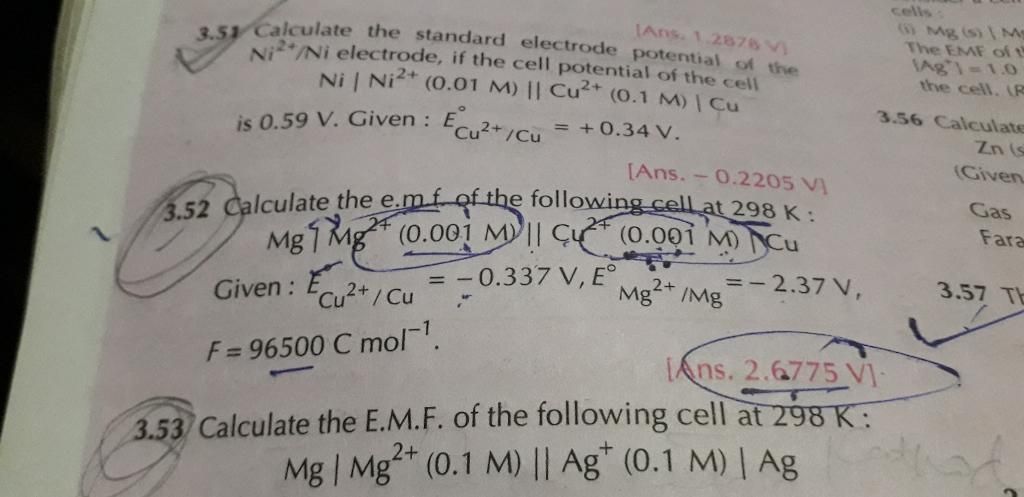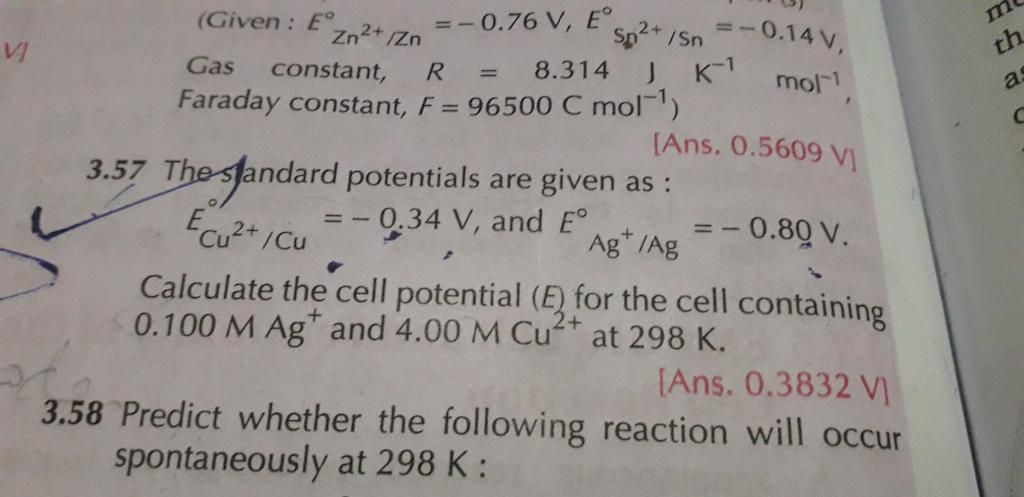CBSE Class 12-science Answered
calculate the mass of silver deposited from silver nitrate solution by a current of 2A flowing for 30 minutes
Asked by chaudharij608 | 03 Jul, 2019, 03:07: PM
Given:
Molar Mass of Ag = 108 g/mol
1F = 96500 C mol−1
1F = 96500 C mol−1
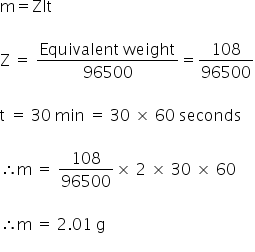
Answered by Ramandeep | 03 Jul, 2019, 03:35: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by avaneesh5116 | 07 Aug, 2020, 05:24: PM
CBSE 12-science - Chemistry
Asked by harshpareek696 | 01 Aug, 2020, 04:01: PM
CBSE 12-science - Chemistry
Asked by sulaikhasulu393 | 22 Jun, 2020, 08:52: AM
CBSE 12-science - Chemistry
Asked by vasudesetti123 | 23 May, 2020, 08:13: PM
CBSE 12-science - Chemistry
Asked by sakthisivasakthi1978 | 31 Oct, 2019, 09:53: PM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 19 Aug, 2019, 11:14: PM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 19 Aug, 2019, 11:13: PM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 19 Aug, 2019, 11:12: PM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 19 Aug, 2019, 11:11: PM
CBSE 12-science - Chemistry
Asked by jain.pradeep | 19 Jul, 2019, 09:49: PM